ROS1-positive non-small cell lung cancer (NSCLC) is a distinct molecular subtype of lung cancer characterized by gene rearrangements involving the ROS1 proto-oncogene. These rearrangements lead to constitutive activation of the ROS1 tyrosine kinase domain, driving oncogenic signaling that promotes cellular proliferation and survival.
Although ROS1 rearrangements occur in only approximately 1–2% of NSCLC cases, their clinical significance is profound due to the availability of targeted therapies that have revolutionized treatment outcomes.
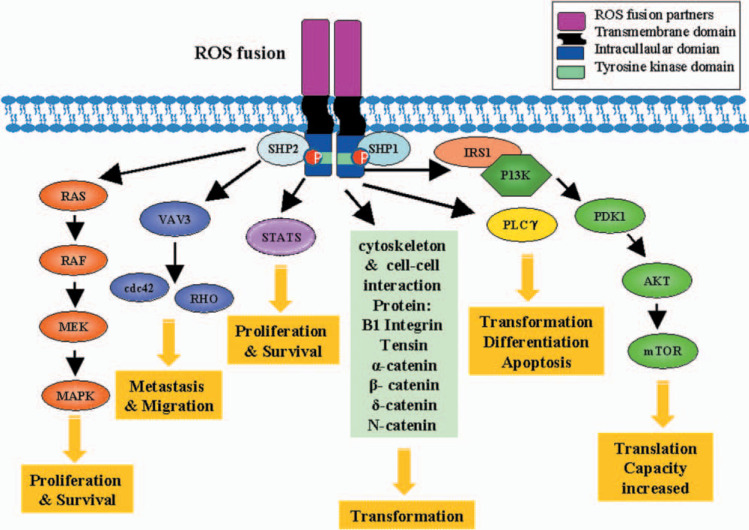
Molecular Basis: ROS1 Rearrangements and Oncogenesis
The ROS1 gene, located on chromosome 6q22, typically fuses with various partner genes such as CD74, SDC4, EZR, TPM3, and others. This fusion leads to the formation of a chimeric oncogene that activates downstream signaling cascades:
These pathways collectively drive uncontrolled tumor cell proliferation and resistance to apoptosis, underscoring the oncogenic potential of ROS1 fusions.
Epidemiology and Patient Demographics
Patients with ROS1-positive NSCLC often share specific demographic and clinical characteristics:
- Typically diagnosed in younger, non-smoking individuals
- Most cases are adenocarcinomas
- Higher prevalence in Asian populations
- Found in approximately 1–2% of all NSCLC cases
Due to its rarity, comprehensive molecular profiling is essential to identify ROS1 fusions and ensure appropriate treatment selection.
Diagnostic Approach and Molecular Testing
Accurate detection of ROS1 rearrangements is critical for therapeutic decision-making. Recommended diagnostic methodologies include:
1. Next-Generation Sequencing (NGS)
- Detects known and novel fusion partners with high sensitivity
- Can simultaneously assess multiple oncogenic drivers
2. Fluorescence In Situ Hybridization (FISH)
- Historically the gold standard for ROS1 detection
- Identifies gene rearrangement but not fusion partners
3. Immunohistochemistry (IHC)
- Cost-effective screening method
- Positive results must be confirmed by NGS or FISH
4. Reverse Transcriptase PCR (RT-PCR)
- High specificity but limited to known fusions
Tissue and liquid biopsy samples are both acceptable sources for molecular analysis. Ensuring adequate sample quality is vital for accurate detection.
Clinical Features and Disease Progression
ROS1-positive NSCLC often presents with advanced-stage disease and shares similarities with other oncogene-driven tumors:
- High incidence of brain metastases
- Indolent early progression followed by rapid systemic dissemination
- Responsive to TKIs but prone to resistance development
Symptoms may include chronic cough, dyspnea, chest pain, hemoptysis, and unintended weight loss. The presence of metastases can lead to neurologic symptoms or bone pain.
Targeted Therapies for ROS1-Positive NSCLC
Crizotinib
- First FDA-approved TKI for ROS1-positive NSCLC
- Demonstrated an objective response rate (ORR) of ~72%
- Median progression-free survival (PFS) of 15–19 months
- Limited CNS penetration
Entrectinib
- FDA-approved for ROS1-positive NSCLC with CNS activity
- Higher efficacy in patients with brain metastases
- ORR of ~77%, median PFS around 19 months
Lorlatinib, Repotrectinib, Taletrectinib
- Under investigation or granted breakthrough therapy designations
- Designed to overcome crizotinib resistance and improve CNS penetration
Comparative Overview:
| Therapy | CNS Penetration | ORR (%) | Median PFS (months) |
|---|---|---|---|
| Crizotinib | Low | ~72 | 15–19 |
| Entrectinib | High | ~77 | ~19 |
| Lorlatinib | Very High | TBD | TBD |
Mechanisms of Resistance
Resistance to ROS1-targeted therapies is an emerging clinical challenge. Mechanisms include:
- Secondary mutations in ROS1 kinase domain (e.g., G2032R)
- Bypass pathway activation (e.g., EGFR, KRAS, MET)
- Phenotypic transformation to small-cell lung cancer
Rebiopsy and repeat molecular testing upon disease progression are essential to guide second-line therapy.
Management of CNS Metastases
Brain metastases are common in ROS1-positive NSCLC, requiring therapies with high blood-brain barrier permeability. Entrectinib and investigational agents such as lorlatinib and repotrectinib show promising CNS activity.
Adjunct strategies include:
- Stereotactic radiosurgery (SRS)
- Whole-brain radiation therapy (WBRT) for multifocal lesions
- Supportive corticosteroids for symptom management
Prognosis and Survival Outcomes
With the advent of ROS1-directed therapies, patients with ROS1-positive NSCLC exhibit significantly improved survival metrics:
- Median overall survival (OS): 51–62 months in treated patients
- High initial response rates to TKIs
- Long-term control possible with sequential TKI use and CNS management
However, the prognosis remains guarded without molecularly targeted therapy due to the aggressive nature of the disease upon progression.
Future Directions and Ongoing Research
Key areas of investigation include:
- Next-generation ROS1 inhibitors (e.g., taletrectinib, zidesamtinib)
- Combination therapies targeting multiple resistance pathways
- Minimal residual disease monitoring via circulating tumor DNA (ctDNA)
- Early screening programs in high-risk populations
Clinical trials continue to refine the treatment landscape, aiming to extend survival and improve quality of life.
ROS1-positive non-small cell lung cancer represents a highly actionable molecular subset with remarkable responsiveness to targeted therapy. Precision diagnostics and continuous monitoring are vital to optimize outcomes. As novel agents and resistance strategies evolve, multidisciplinary management and timely molecular profiling remain foundational to improving survival for patients with ROS1-driven lung cancer.