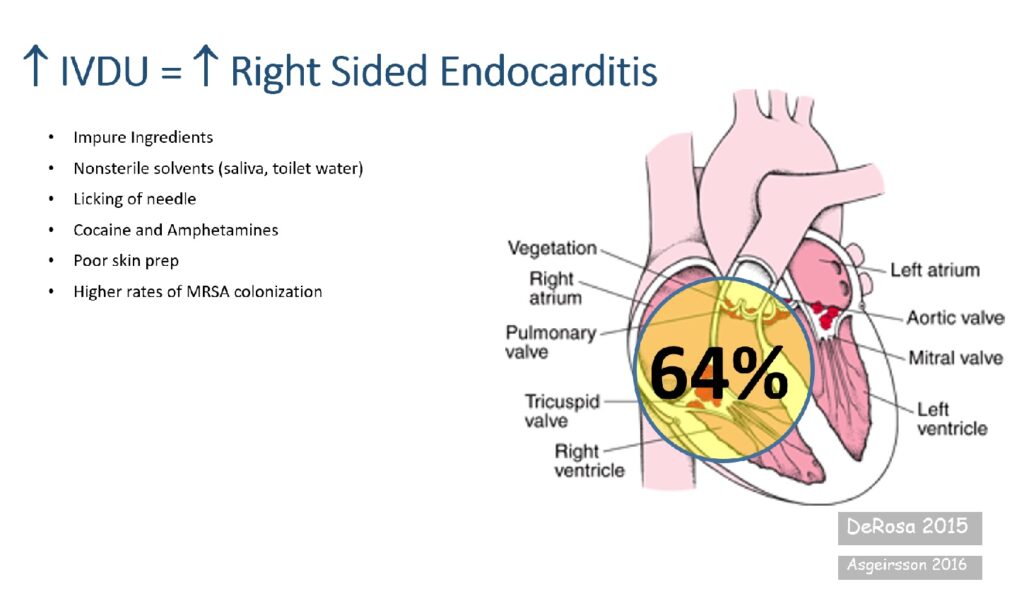
Right-sided Staphylococcus aureus endocarditis is a serious and increasingly prevalent form of infective endocarditis, primarily affecting the tricuspid valve. It is particularly associated with intravenous drug use (IVDU), indwelling catheters, and prosthetic cardiac devices. The pathogenicity of S. aureus, especially methicillin-resistant strains (MRSA), results in rapid valve destruction, septic embolization, and significant morbidity if untreated.
Pathogenesis and Risk Factors
Mechanism of Infection
Staphylococcus aureus, a Gram-positive coccus, adheres to cardiac endothelium via surface proteins that bind fibrinogen and fibronectin. In right-sided endocarditis, the infection typically begins on the tricuspid valve and extends into surrounding cardiac tissue.
Primary Risk Factors
- Intravenous drug use (most common cause)
- Intravascular catheters or pacemakers
- Congenital heart disease involving right-sided valves
- Immunosuppression or neutropenia
- Preexisting valvular disease
- Prosthetic tricuspid valves
Clinical Presentation and Symptoms
Right-sided endocarditis presents with features that may differ from left-sided involvement due to pulmonary embolization of vegetations.
Common Symptoms
| Symptom | Description |
|---|---|
| Fever | Persistent, often the initial presenting complaint |
| Cough & Chest Pain | Due to pulmonary emboli |
| Dyspnea | From septic pulmonary embolism |
| Hemoptysis | Indicates pulmonary infarcts |
| Fatigue & Malaise | Generalized systemic involvement |
| Heart Murmur | New or changing systolic murmur over tricuspid area |
Physical Findings
- Pleuritic chest pain
- Pulmonary crackles
- Signs of right heart failure (jugular venous distention, peripheral edema)
- Tricuspid regurgitation murmur (accentuated during inspiration)
Microbiological Profile
Staphylococcus aureus is the predominant pathogen in right-sided endocarditis.
- Methicillin-sensitive S. aureus (MSSA): More common in non-hospital-acquired infections
- Methicillin-resistant S. aureus (MRSA): Frequent in IVDU and healthcare-associated infections
Diagnostic Approach
Timely and accurate diagnosis is essential for effective treatment and prevention of complications.
Blood Cultures
- Obtain at least 3 sets of blood cultures before initiating antibiotics
- Positive cultures for S. aureus confirm the etiology
Echocardiography
| Modality | Utility |
|---|---|
| Transthoracic Echo (TTE) | Initial assessment; useful in detecting large vegetations |
| Transesophageal Echo (TEE) | Higher sensitivity; recommended if TTE is negative and suspicion persists |
Modified Duke Criteria
A standardized diagnostic framework using major and minor clinical, microbiological, and echocardiographic findings.
Complications of Right-Sided Endocarditis
Right-sided endocarditis is prone to septic embolization to the lungs and valvular damage.
Major Complications
- Septic pulmonary emboli
- Pulmonary infarction or abscess
- Tricuspid valve destruction
- Right heart failure
- Persistent bacteremia
- Rarely, systemic embolization in cases of intracardiac shunt
Antimicrobial Treatment
Empirical Therapy (Prior to Culture Results)
- Vancomycin: 15–20 mg/kg IV every 8–12 hours (for MRSA coverage)
- Cefazolin or Nafcillin: For MSSA if suspected in community-acquired cases
Targeted Therapy (Post Culture Identification)
| Pathogen | Recommended Regimen | Duration |
|---|---|---|
| MSSA | Nafcillin or Cefazolin | 4–6 weeks |
| MRSA | Vancomycin or Daptomycin | 6 weeks |
| Polymicrobial | Combination therapy guided by susceptibility | Varies |
Shorter 2-week regimens may be considered in uncomplicated cases of tricuspid valve MSSA endocarditis with rapid clinical response and no metastatic infection.
Surgical Intervention
Surgical treatment is considered in patients who fail medical therapy or develop complications.
Indications for Surgery
- Refractory right heart failure due to tricuspid regurgitation
- Persistent bacteremia despite appropriate antibiotics
- Large vegetations (>20 mm) with recurrent pulmonary emboli
- Fungal or highly resistant organism infection
Valve repair is preferred over replacement, when feasible, to minimize prosthetic-related complications in younger patients or those with active IVDU.
Prognosis and Long-Term Management
With early diagnosis and appropriate therapy, right-sided staphylococcal endocarditis carries a better prognosis than left-sided disease. However, recurrences are common, particularly in individuals with ongoing risk factors such as IVDU.
Prognostic Factors
- Pathogen resistance (MRSA associated with poorer outcomes)
- Size of vegetations
- Response to antibiotics within 7 days
- Comorbidities (HIV, liver disease)
Follow-Up Recommendations
- Serial echocardiograms to assess vegetation resolution
- Repeated blood cultures to confirm eradication
- Education on cessation of IVDU
- Long-term care for heart failure, if present
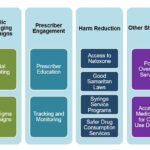
Prevention and Public Health Measures
Harm Reduction in IVDU
- Needle exchange programs
- Supervised injection facilities
- Addiction counseling and rehabilitation services
Infection Control in Healthcare Settings
- Sterile technique during catheter insertion
- Timely removal of unnecessary intravascular devices
- Hand hygiene and decolonization protocols for S. aureus
Right-sided Staphylococcus aureus endocarditis is a distinct clinical entity with unique diagnostic and management considerations. Early recognition, microbiological confirmation, and tailored antibiotic therapy are paramount. With appropriate intervention, outcomes are favorable, though prevention through harm reduction remains a cornerstone in addressing recurrent cases.