Rivaroxaban, a direct Factor Xa inhibitor, is widely used for stroke prevention in atrial fibrillation, treatment of venous thromboembolism (VTE), and postoperative thromboprophylaxis. While effective in reducing thromboembolic events, its anticoagulant effect may require urgent reversal in the setting of major bleeding, emergency surgery, or traumatic injury. The lack of routine monitoring and short half-life are beneficial, but in life-threatening events, prompt intervention is essential.
Pharmacokinetics of Rivaroxaban Relevant to Reversal
- Onset of Action: 2–4 hours
- Half-Life: 5–9 hours (young), 11–13 hours (elderly)
- Renal Clearance: ~33%
- Hepatic Metabolism: CYP3A4, CYP2J2 pathways
Reversal decisions must consider timing of the last dose, renal function, and bleeding severity.
Indications for Rivaroxaban Reversal
- Life-threatening or uncontrolled bleeding (e.g., intracranial hemorrhage, GI bleed)
- Urgent surgical or invasive procedures
- Overdose with high bleeding risk
- Prolonged anticoagulant effect due to renal or hepatic dysfunction
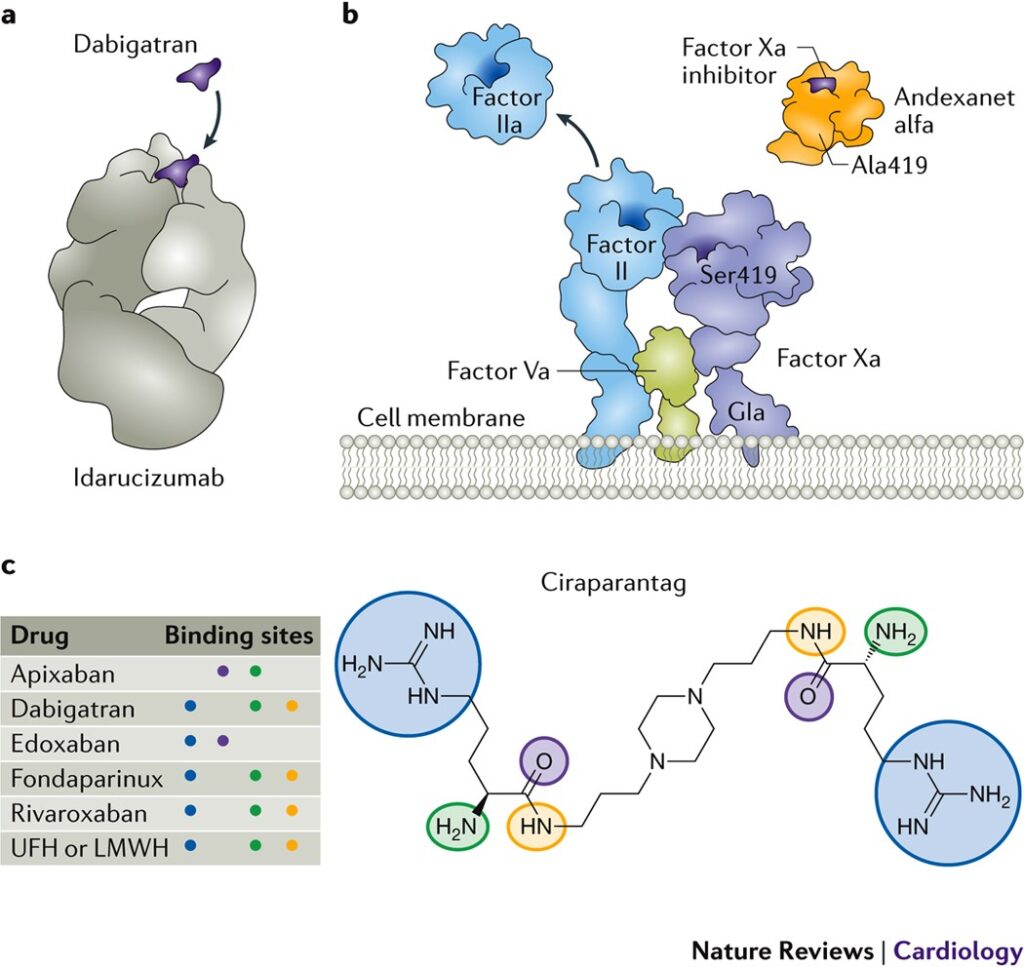
First-Line Reversal Agent: Andexanet Alfa
Andexanet alfa is a recombinant modified human Factor Xa decoy protein that binds and neutralizes the anticoagulant effects of rivaroxaban.
Mechanism of Action
It acts by sequestering free rivaroxaban, thereby restoring endogenous Factor Xa activity and thrombin generation.
Dosing Protocol
Dosing depends on rivaroxaban dose and timing of last administration.
| Rivaroxaban Dose | Time Since Last Dose | Andexanet Alfa Regimen |
|---|---|---|
| ≤10 mg | <7 hours or unknown | Low dose (400 mg IV bolus + 4 mg/min x 120 min) |
| >10 mg or unknown | <7 hours | High dose (800 mg IV bolus + 8 mg/min x 120 min) |
| >7 hours | Consider clinical observation or PCC if needed |
Efficacy and Limitations
- Hemostatic efficacy: ~80% in major bleeding
- Limited data for non-major bleeds
- High cost and limited availability
- Not approved in all countries
Alternative: Prothrombin Complex Concentrates (PCCs)
In settings where andexanet alfa is unavailable, 4-factor PCCs (containing Factors II, VII, IX, and X) can be used off-label to reverse rivaroxaban effects.
Suggested Dosing
- 25–50 IU/kg IV, typically 2,000–3,000 IU total
- Administer within minutes of identifying life-threatening hemorrhage
Mechanism
PCCs replenish vitamin K-dependent clotting factors, bypassing the inhibition of Factor Xa to restore thrombin generation.
Considerations
- May increase thrombotic risk
- Not a direct antidote, but effective in restoring hemostasis
- Monitor clinical response and coagulation markers if available
Role of Activated Charcoal in Recent Ingestion
In cases of recent overdose (within 2 hours) of rivaroxaban, activated charcoal may reduce systemic absorption.
Recommended Use
- 50 g orally within 2 hours of ingestion
- Can be combined with other reversal strategies
Laboratory Monitoring in Reversal Scenarios
Although routine monitoring of rivaroxaban is not required, specific assays can guide reversal:
| Test | Interpretation |
|---|---|
| Anti-Factor Xa assay | Quantifies rivaroxaban activity |
| PT/INR | May be elevated, not specific |
| aPTT | Variable; not reliable |
| Thrombin generation | May help in assessing reversal effect |
Surgical and Invasive Procedures
In patients requiring emergency surgery, reversal should be tailored to urgency and bleeding risk:
| Procedure Type | Action |
|---|---|
| Elective | Delay until drug eliminated (~24 hrs) |
| Urgent/High-Risk | Administer andexanet alfa or PCC |
| Low-Risk | Consider no reversal if bleeding risk minimal |
Clinical Scenarios and Recommendations
Intracranial Hemorrhage
- Immediate reversal with andexanet alfa or PCC
- Consider neurosurgical intervention after stabilization
Gastrointestinal Bleed
- Hemodynamic support, endoscopy
- Reversal agents for uncontrolled or severe bleeds
Overdose without Bleeding
- Monitor renal function, coagulation
- Consider activated charcoal and observation
Adverse Events and Risk Management
| Reversal Agent | Key Risks |
|---|---|
| Andexanet Alfa | Thromboembolic events, infusion reactions |
| PCCs | Venous thromboembolism, DIC |
| Activated Charcoal | Aspiration, especially in obtunded patients |
Monitor patients closely post-reversal for thrombosis, rebleeding, and hemodynamic stability.
Frequently Asked Questions:
Is there an antidote for rivaroxaban?
Yes, andexanet alfa is the specific reversal agent approved for direct Factor Xa inhibitors including rivaroxaban.
What if andexanet alfa is not available?
Administer 4-factor PCC at 25–50 IU/kg IV to reverse anticoagulation.
How long should rivaroxaban be withheld after reversal?
Reinitiation depends on bleeding control, typically 48–72 hours after stabilization.
Does dialysis remove rivaroxaban?
No, due to high protein binding, rivaroxaban is not dialyzable.
When is activated charcoal effective for rivaroxaban overdose?
Within 2 hours of ingestion for optimal gastrointestinal decontamination.
Effective reversal of rivaroxaban anticoagulation is critical in managing life-threatening bleeding or urgent surgical interventions. Andexanet alfa, when available, provides a targeted and rapid reversal. In its absence, PCCs remain a viable alternative. Clinical judgment, supported by laboratory data and individualized patient factors, should drive the choice and timing of reversal strategy. Incorporating structured protocols can significantly enhance outcomes in anticoagulant-associated emergencies.