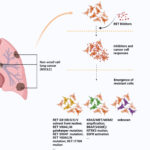
RET fusion-positive thyroid cancer represents a distinct molecular subset of differentiated thyroid cancers, predominantly papillary thyroid carcinoma (PTC) and poorly differentiated thyroid carcinoma (PDTC). RET (rearranged during transfection) is a proto-oncogene encoding a receptor tyrosine kinase involved in cell proliferation and survival. RET fusions occur when the RET kinase domain becomes aberrantly activated through fusion with partner genes, most commonly CCDC6 or NCOA4.
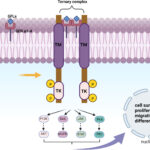
RET fusions lead to constitutive kinase activation independent of ligand binding, which drives oncogenesis via downstream pathways:
RET fusions are present in approximately 10-20% of sporadic PTCs and up to 60% of radiation-associated thyroid cancers, particularly in younger individuals and those with minimal exposure to ionizing radiation.
Epidemiology and Clinical Features
RET fusion-positive thyroid cancers are more frequently observed in:
- Children and adolescents
- Patients with a history of radiation exposure
- Tumors with aggressive histologic features, such as tall-cell variant of PTC
Clinically, these tumors often present with:
- Rapid neck mass enlargement
- Cervical lymphadenopathy
- Hoarseness or dysphagia in advanced cases
- High rates of regional and, occasionally, distant metastases
Despite their aggressive behavior, these tumors often retain iodine avidity, especially in earlier stages.
Diagnostic Approach to RET Fusion-Positive Thyroid Cancer
Accurate detection of RET fusions is essential for personalized therapy. Diagnosis includes:
Histopathological Evaluation
Initial thyroid nodule assessment involves fine-needle aspiration (FNA) followed by cytologic analysis using the Bethesda classification. Suspicious or malignant cytology warrants surgical excision and molecular testing.
Molecular Testing
RET fusions are best identified through:
- Next-Generation Sequencing (NGS): Gold standard for identifying RET fusion partners
- Reverse Transcription PCR (RT-PCR): Useful for known RET partners but lacks comprehensiveness
- Fluorescence In Situ Hybridization (FISH): Detects RET rearrangements but does not identify fusion partners
Liquid biopsy may complement tissue testing, particularly in metastatic or recurrent cases.
Targeted Therapy for RET Fusion-Positive Thyroid Cancer
Conventional treatment for differentiated thyroid cancer includes surgery, radioactive iodine (RAI) therapy, and thyroid-stimulating hormone (TSH) suppression. However, RET fusion-positive tumors, especially when metastatic or refractory to RAI, require precision-targeted systemic therapy.
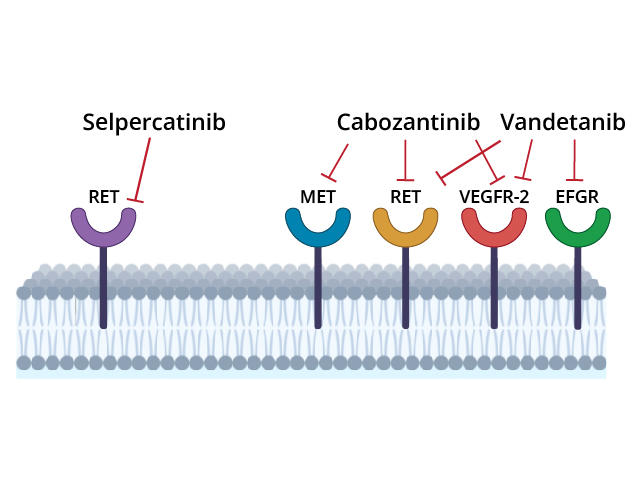
Selpercatinib
- Highly selective RET kinase inhibitor
- FDA-approved for RET fusion-positive thyroid cancer (including RAI-refractory)
- Demonstrates high overall response rate (ORR ~79%)
- Effective in patients with CNS metastases
- Common adverse effects: dry mouth, hypertension, elevated liver enzymes
Pralsetinib
- Alternative selective RET inhibitor with FDA approval
- Comparable ORR (~60-70%) in treatment-naïve and previously treated patients
- Shows durable disease control with manageable toxicity
- Side effects: fatigue, neutropenia, constipation
Both agents are recommended as first-line treatments in metastatic or RAI-refractory RET fusion-positive thyroid cancer.
Resistance and Disease Progression
Acquired resistance to RET inhibitors can emerge through:
- Secondary RET mutations (e.g., solvent front mutations like G810R/S/C)
- Activation of bypass signaling pathways (e.g., MET or KRAS)
- Histologic dedifferentiation to more aggressive subtypes
Investigational therapies and next-generation RET inhibitors are being developed to overcome these resistance mechanisms.
Prognosis and Survival Outcomes
When treated with selective RET inhibitors, patients with advanced RET fusion-positive thyroid cancer have significantly improved outcomes compared to standard therapies:
- Median progression-free survival (PFS): Up to 20 months (selpercatinib)
- Overall survival (OS): Not yet reached in ongoing clinical trials
- Durable disease control and potential re-sensitization to RAI in some cases
The integration of targeted therapy into clinical practice has redefined prognosis for this molecular subtype.
Surveillance and Follow-Up
Long-term management includes:
- Regular imaging (neck ultrasound, CT, or PET-CT for distant metastases)
- Serum thyroglobulin (Tg) monitoring
- Repeat molecular testing in the event of progression to identify resistance mutations
- TSH suppression and, where applicable, consideration for re-treatment with RAI if redifferentiation occurs post-RET inhibition
Emerging Research and Future Therapeutic Strategies
Ongoing clinical trials are investigating:
- Combination therapies (RET inhibitors with MEK or immune checkpoint inhibitors)
- Adjuvant RET-targeted treatment in high-risk post-surgical patients
- Novel RET inhibitors with broader resistance mutation coverage
- Circulating tumor DNA (ctDNA) for minimal residual disease monitoring
Advancements in molecular oncology continue to refine treatment personalization and improve patient outcomes.
Frequently Asked Questions:
What is RET fusion-positive thyroid cancer?
It is a subtype of thyroid cancer driven by a genetic fusion involving the RET gene, leading to uncontrolled cell growth.
How is RET fusion detected?
RET fusions are identified through molecular tests such as NGS, FISH, or RT-PCR on biopsy samples.
Is RET fusion more common in certain patients?
Yes, it is more prevalent in children, young adults, and those with a history of radiation exposure.
What are the best treatments for RET fusion-positive thyroid cancer?
Selective RET inhibitors like selpercatinib and pralsetinib are the most effective treatments for advanced or RAI-refractory cases.
Can RET fusion-positive thyroid cancer become resistant to treatment?
Yes, resistance can develop through secondary mutations or bypass signaling, requiring alternative therapies.