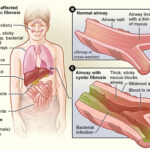
Respiratory cystic fibrosis (CF) is a life-limiting, autosomal recessive disorder caused by mutations in the CFTR gene, resulting in defective chloride ion transport across epithelial cells. This leads to the production of dehydrated, viscous mucus that obstructs airways and promotes chronic pulmonary infections. Among the pathogens involved, Pseudomonas aeruginosa is the most prominent and persistent, contributing significantly to morbidity, lung function decline, and mortality in CF patients.
The Role of Pseudomonas aeruginosa in Cystic Fibrosis Lung Infections
Pathogenic Characteristics
Pseudomonas aeruginosa is an opportunistic, Gram-negative bacterium known for its capacity to colonize the lungs of individuals with cystic fibrosis. Once established, it adapts to the host environment, forming biofilms and transitioning to a mucoid phenotype that enhances resistance to antibiotics and host immune responses.
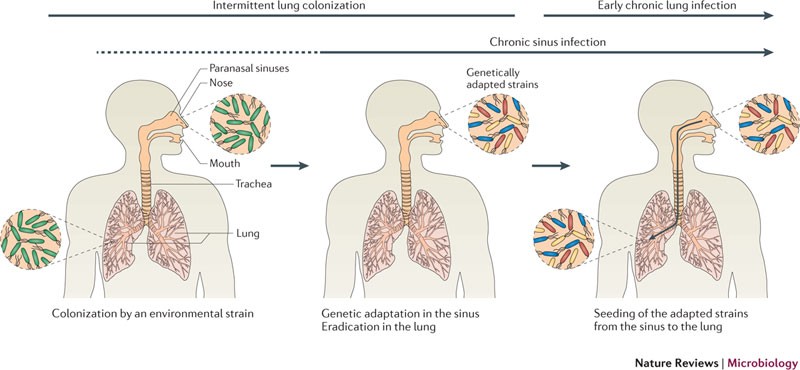
Chronic Colonization
- Initial Infection: In early stages, P. aeruginosa appears intermittently and is susceptible to eradication with aggressive antibiotic therapy.
- Chronic Colonization: Over time, repeated exposure leads to chronic colonization characterized by the presence of mucoid strains, which are more difficult to eradicate and contribute to persistent inflammation and progressive airway damage.
Clinical Consequences of Pseudomonas aeruginosa in CF
Accelerated Lung Function Decline
Patients with chronic P. aeruginosa colonization demonstrate significantly faster declines in FEV₁ (Forced Expiratory Volume in one second), leading to earlier respiratory failure.
Increased Frequency of Exacerbations
Chronic infection promotes acute pulmonary exacerbations, marked by increased cough, sputum production, dyspnea, and systemic symptoms. These episodes often require hospitalization and intravenous antibiotic therapy.
Antibiotic Resistance
Biofilm formation and the mucoid phenotype contribute to multidrug resistance, making treatment increasingly complex and requiring combination or inhaled antibiotic therapies.
Diagnosis and Monitoring of P. aeruginosa Colonization in CF
Routine Microbiological Surveillance
- Sputum Culture: The standard method for detecting P. aeruginosa colonization.
- Oropharyngeal Swabs: Used for patients unable to expectorate sputum, especially in children.
- Bronchoalveolar Lavage (BAL): Employed in select cases to assess lower respiratory tract colonization.
Biomarkers and Imaging
- Serum Inflammatory Markers: Elevated CRP and neutrophil counts often accompany exacerbations.
- High-Resolution CT Scans: Reveal bronchiectasis and mucus plugging associated with chronic infection.
Evidence-Based Treatment of CF with P. aeruginosa Infection
Eradication Protocols (Early Infection)
- Inhaled Tobramycin: Administered twice daily for 28 days as first-line therapy.
- Inhaled Colistin or Aztreonam Lysine: Alternatives for tobramycin-resistant strains or intolerance.
Chronic Suppression Therapy
- Long-term Inhaled Antibiotics: Rotating or continuous therapy with tobramycin, aztreonam, or colistin.
- Oral Ciprofloxacin: Used in combination with inhaled therapy for acute flares or mild colonization.
- Intravenous Therapy: Reserved for exacerbations or severe disease requiring hospitalization.
Adjunctive Therapies
- Airway Clearance Techniques: Daily chest physiotherapy, high-frequency chest wall oscillation, and positive expiratory pressure therapy.
- Anti-inflammatory Agents: Azithromycin used for its immunomodulatory effects.
- CFTR Modulators: Ivacaftor, lumacaftor/ivacaftor, and elexacaftor/tezacaftor/ivacaftor can improve lung function and reduce infection frequency.
Preventing Pseudomonas aeruginosa Acquisition
- Environmental Control: Avoiding contaminated water sources (e.g., hot tubs, humidifiers).
- Infection Control Protocols: Strict hygiene practices in clinical and home settings.
- Prophylactic Antibiotics: Occasionally used post-lung transplant or in high-risk scenarios.
Long-Term Prognosis and Research Advances
While chronic P. aeruginosa colonization significantly worsens outcomes, early intervention and adherence to eradication and suppression regimens can slow disease progression. Ongoing research into bacteriophage therapy, novel inhaled antibiotics, and anti-biofilm agents holds promise for more effective treatment modalities in the future.