Recurrent Clostridioides difficile infection (CDI) presents a growing healthcare challenge worldwide. It is characterized by the reappearance of symptomatic infection within eight weeks following the completion of appropriate antibiotic therapy. Recurrent CDI significantly increases morbidity, healthcare costs, and the risk of further recurrences, necessitating a comprehensive and sustained management approach.
Causes and Risk Factors for Recurrent CDI
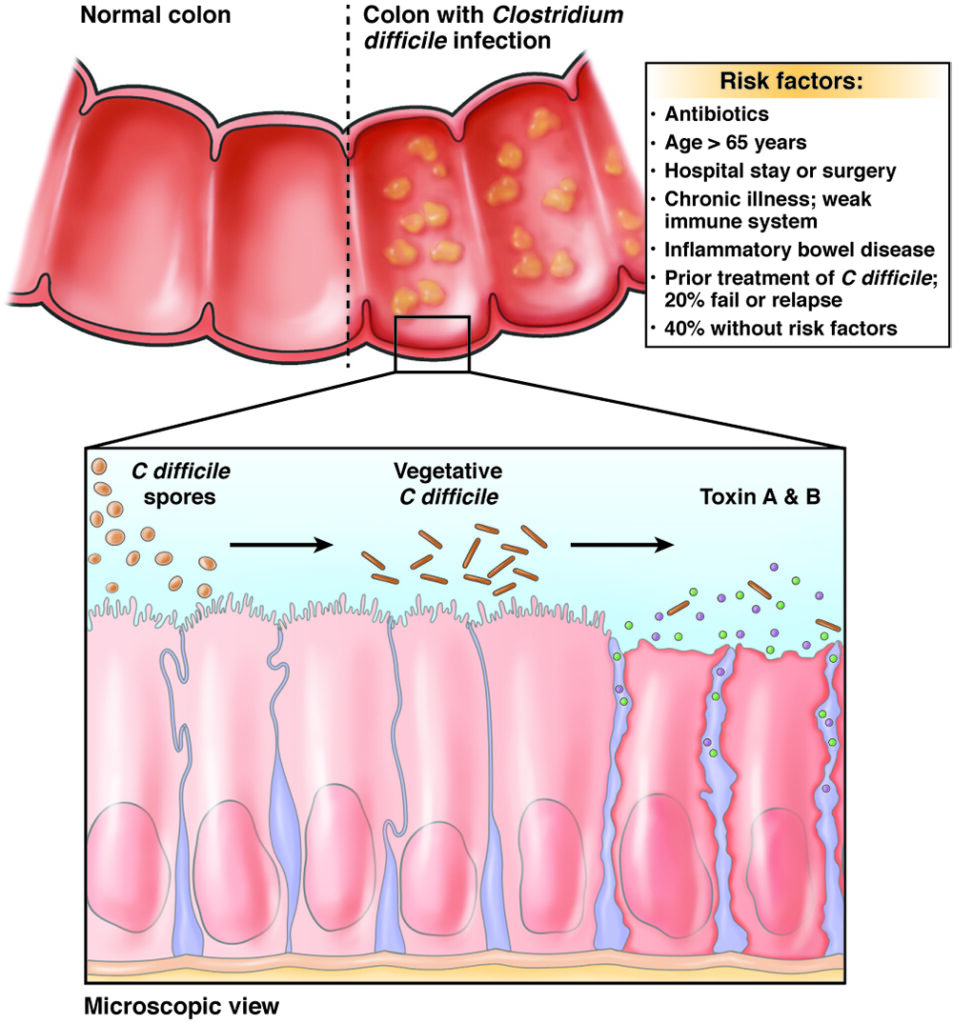
Recurrent CDI arises due to two principal mechanisms: relapse of the original infection due to persistent spores and reinfection with a new strain. Several risk factors predispose patients to recurrence:
- Age over 65 years
- Use of broad-spectrum antibiotics
- Proton pump inhibitor (PPI) use
- Immunocompromised states
- Prior episodes of CDI
- Underlying gastrointestinal disorders
Impaired gut microbiota recovery following antibiotic therapy creates a vulnerable environment, favoring C. difficile overgrowth and toxin production.
Pathogenesis of Recurrent CDI
The pathogenic cycle begins with disruption of normal colonic flora, followed by colonization by toxigenic C. difficile strains. Toxin A and Toxin B production leads to inflammation, diarrhea, and colonic mucosal damage.
Clinical Presentation of Recurrent CDI
Recurrent CDI typically mirrors the initial infection, presenting with:
- Watery diarrhea (≥3 unformed stools in 24 hours)
- Fever
- Abdominal cramping and pain
- Nausea
- Leukocytosis
- Dehydration
Persistent or escalating symptoms warrant immediate reassessment and tailored management to prevent complications such as toxic megacolon or sepsis.
Diagnostic Approach for Recurrent CDI
Accurate and timely diagnosis of recurrent CDI requires a combination of clinical assessment and laboratory testing:
- Stool Testing:
- Nucleic Acid Amplification Test (NAAT): High sensitivity but cannot differentiate colonization from infection.
- Glutamate Dehydrogenase (GDH) and Toxin Assays: Employed together for greater diagnostic accuracy.
- Colonoscopy: Reserved for atypical presentations or when ischemic colitis or inflammatory bowel disease flare is suspected.
Testing is recommended only in patients with new or worsening diarrhea and risk factors to avoid overdiagnosis.
Evidence-Based Management of Recurrent CDI
First Recurrence
- Preferred Therapy: Oral vancomycin in a tapered and pulsed regimen or fidaxomicin.
- Alternative Therapy: Standard 10-day course of fidaxomicin, particularly in patients at high risk for further recurrence.
Multiple Recurrences
- Vancomycin Taper or Fidaxomicin: Another round of tapered vancomycin or fidaxomicin.
- Fecal Microbiota Transplantation (FMT): Highly effective following multiple failed antibiotic therapies, restoring healthy gut flora.
Fecal Microbiota Transplantation (FMT) in Recurrent CDI
FMT is considered the most efficacious therapy for recurrent CDI, boasting cure rates exceeding 85%. It involves the transfer of screened donor stool into the patient’s colon via colonoscopy, enema, or capsule.
Mechanism of Action
FMT works by restoring the diversity and functionality of gut microbiota, thereby suppressing C. difficile colonization and toxin production.
Procedure Overview
- Donor Selection: Rigorous medical screening to minimize infectious risks.
- Sample Processing: Preparation and homogenization under sterile conditions.
- Administration Routes: Colonoscopy (most effective), nasogastric tube, or oral capsules.
Post-procedure, patients often experience rapid resolution of symptoms within days.
Prevention Strategies for Recurrent CDI
Effective prevention is multifactorial and includes:
Antibiotic Stewardship
- Restrictive use of high-risk antibiotics such as clindamycin, fluoroquinolones, and cephalosporins.
- Shortest effective duration for necessary antibiotic therapy.
Infection Control Measures
- Hand Hygiene: Hand washing with soap and water (C. difficile spores are alcohol-resistant).
- Contact Precautions: Use of gloves and gowns during patient care.
- Environmental Cleaning: Use of sporicidal agents for surface disinfection.
Probiotic Use
While evidence is evolving, specific probiotics may confer protective benefits during antibiotic therapy, though they should be used cautiously, particularly in immunocompromised patients.
Vaccination
Vaccines targeting C. difficile toxins are under development and may offer future preventive avenues.
Complications of Untreated or Poorly Managed Recurrent CDI
- Severe Colitis: Requiring colectomy in extreme cases.
- Toxic Megacolon: Life-threatening colonic dilation.
- Sepsis: Secondary to bacterial translocation.
- Chronic Diarrhea and Malnutrition: Impairing quality of life.
Recognizing early signs of recurrence and initiating prompt, evidence-based interventions are vital to mitigate risks.
Recurrent Clostridioides difficile infection remains a complex and challenging condition, necessitating an integrated approach encompassing meticulous diagnosis, appropriate therapeutic regimens, and preventive strategies. By optimizing patient care pathways and leveraging emerging therapies like FMT, we can significantly reduce recurrence rates, enhance patient outcomes, and alleviate the healthcare burden associated with CDI.