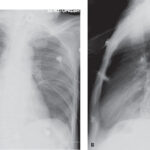
Pseudomonas aeruginosa septicemia is a rapidly progressing and often fatal bloodstream infection caused by a gram-negative, opportunistic pathogen. Known for its robust resistance mechanisms and nosocomial prevalence, this bacterium presents a formidable challenge in critical care settings, especially among immunocompromised individuals, burn victims, and patients with prolonged hospitalization or invasive devices.
Epidemiology and High-Risk Patient Groups
Pseudomonas septicemia accounts for a significant proportion of healthcare-associated bloodstream infections (BSIs), particularly in:
- Intensive care unit (ICU) patients
- Individuals undergoing chemotherapy or organ transplantation
- Burn patients with compromised skin barriers
- Neonates and elderly populations
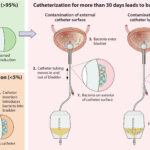
- Patients with indwelling catheters or mechanical ventilation
Hospital environments, broad-spectrum antibiotic exposure, and invasive procedures increase colonization and risk of bacteremia.
Pathogenesis and Mechanisms of Sepsis Development
Following colonization, P. aeruginosa can translocate into the bloodstream via compromised mucosal barriers, contaminated catheters, or infected wounds. It activates a cascade of inflammatory responses leading to systemic infection, often progressing to septic shock.
Stages of Pseudomonas Septicemia
Virulence factors such as exotoxin A, elastase, phospholipase C, and pyocyanin contribute to tissue injury, immune evasion, and vascular leakage.
Clinical Manifestations of Pseudomonas Septicemia
Pseudomonas septicemia typically presents with nonspecific but rapidly escalating symptoms:
- High fever with rigors
- Hypotension and tachycardia
- Altered mental status
- Cold or cyanotic extremities
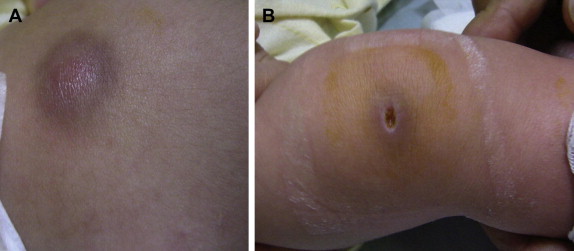
- Skin lesions such as ecthyma gangrenosum
- Signs of organ failure (e.g., oliguria, respiratory distress, jaundice)
In neutropenic or immunosuppressed patients, presentation may be subtle, necessitating aggressive monitoring and low threshold for investigation.
Diagnostic Evaluation and Laboratory Investigations
Blood Cultures and Microbiological Confirmation
- Obtain at least two sets of aerobic and anaerobic blood cultures before initiating antibiotics
- Growth is typically rapid in automated systems
- Gram stain may reveal slender gram-negative rods
- MALDI-TOF or PCR assays provide rapid organism identification
Supportive Laboratory Findings
- Elevated procalcitonin, C-reactive protein (CRP), and lactate
- Leukocytosis or leukopenia with toxic granulation
- Thrombocytopenia and rising creatinine in advanced sepsis
- Positive culture from a secondary site (e.g., urine, wound, respiratory tract)
Antimicrobial Therapy and Resistance Concerns
Empirical Treatment Approach
Prompt initiation of broad-spectrum antibiotics is crucial. Empirical regimens should include two antipseudomonal agents from different classes:
- Antipseudomonal β-lactams: piperacillin-tazobactam, ceftazidime, cefepime
- Carbapenems: meropenem or imipenem (in MDR settings)
- Aminoglycosides: tobramycin or amikacin
- Fluoroquinolones: ciprofloxacin or levofloxacin
De-escalation Based on Sensitivities
Upon culture and sensitivity data, streamline to the most effective agent:
- Susceptible isolates may be treated with monotherapy
- MDR or XDR strains often require combination therapy or novel agents such as ceftolozane-tazobactam, ceftazidime-avibactam, or polymyxins
- Treatment duration ranges from 10 to 14 days, extended in persistent or deep-seated infections
Multidrug Resistance and Therapeutic Challenges
P. aeruginosa demonstrates a high degree of intrinsic and acquired resistance through:
- Beta-lactamase production
- Efflux pump upregulation
- Porin channel mutations
- Biofilm-associated protection on catheter surfaces
Carbapenem resistance in Pseudomonas is particularly concerning, necessitating advanced diagnostic stewardship and infection control.
Supportive and Adjunctive Therapies
- Fluid resuscitation: Guided by lactate clearance and hemodynamic monitoring
- Vasopressors: Norepinephrine is first-line in septic shock
- Renal replacement therapy: For acute kidney injury
- Mechanical ventilation: In cases of respiratory failure
- Glycemic control and nutritional support in ICU settings
Monitoring organ function, adjusting doses based on renal clearance, and daily reassessment of antibiotic need are critical.
Complications and Mortality Risk
Without timely intervention, Pseudomonas aeruginosa septicemia can progress to:
- Disseminated intravascular coagulation (DIC)
- Acute respiratory distress syndrome (ARDS)
- Septic shock and refractory hypotension
- Endocarditis or metastatic abscesses
- Mortality rates exceeding 40–60%, particularly in MDR or delayed-treatment cases
Prevention Strategies in Clinical Settings
- Strict hand hygiene and environmental sanitation
- Sterile insertion and maintenance of catheters and lines
- Removal of unnecessary invasive devices
- Surveillance cultures in high-risk units
- Antibiotic stewardship to reduce selection pressure
- Isolation of colonized or infected individuals where appropriate
Frequently Asked Questions:
Q1. What causes Pseudomonas aeruginosa to enter the bloodstream?
It often enters through wounds, catheters, or compromised mucosal barriers in immunocompromised or critically ill patients.
Q2. How is Pseudomonas septicemia treated?
It requires prompt initiation of intravenous broad-spectrum antibiotics, later tailored to sensitivity patterns, with full supportive care.
Q3. Is Pseudomonas aeruginosa septicemia life-threatening?
Yes, it can lead to rapid deterioration, septic shock, and high mortality, especially in hospital-acquired or resistant cases.
Q4. Can this infection be prevented?
Prevention hinges on infection control, sterile techniques, minimizing invasive devices, and judicious antibiotic use.
Q5. What makes Pseudomonas more dangerous than other bacteria in septicemia?
Its resistance to multiple antibiotics and ability to cause systemic damage through toxins and immune evasion mechanisms.