Von Willebrand’s disease (VWD) is the most common inherited bleeding disorder, caused by quantitative or qualitative defects in von Willebrand factor (VWF). The disease impairs platelet adhesion and factor VIII stabilization, increasing the risk of excessive bleeding during and after surgical procedures. Surgical interventions in patients with VWD require meticulous preoperative evaluation and personalized hemostatic management to prevent complications.
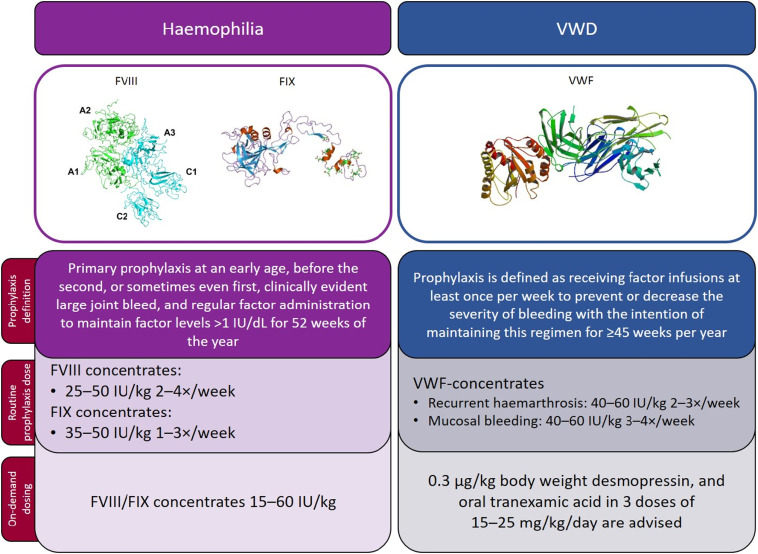
Classification of Von Willebrand’s Disease and Its Surgical Implications
The clinical approach to surgical bleeding prevention depends heavily on the type and severity of VWD:
- Type 1 (Partial quantitative deficiency)
Usually mild; often responds to desmopressin. - Type 2 (Qualitative defects)
Subdivided into 2A, 2B, 2M, and 2N, each with distinct functional abnormalities. Variable response to desmopressin. - Type 3 (Total deficiency of VWF)
Severe form requiring replacement therapy; desmopressin is ineffective.
Correct classification is essential to determine the appropriate perioperative prophylaxis.
Preoperative Assessment: Individualized Risk Stratification
A comprehensive preoperative evaluation must include:
- Detailed bleeding history
- Type and severity of VWD
- Surgical procedure type and bleeding risk
- Laboratory tests:
- VWF:Ag (antigen level)
- VWF:RCo or VWF:GPIbM (activity)
- Factor VIII:C
- VWF multimer analysis (if needed)
- Bleeding time or PFA-100
Desmopressin challenge testing may be conducted prior to surgery to evaluate responsiveness in Types 1 and 2.
Perioperative Hemostatic Management Strategies
Desmopressin (DDAVP)
Indication:
Primarily effective in Type 1 and some Type 2 variants.
Mechanism:
Stimulates release of endogenous VWF and factor VIII.
Administration:
- Intravenous, subcutaneous, or intranasal
- Given 30–90 minutes prior to surgery
Precautions:
- Monitor for hyponatremia and fluid retention
- Ineffective in Type 3 and some 2B or 2N cases
VWF/FVIII Concentrates
Indication:
Preferred for Type 3 and desmopressin non-responders.
Product Types:
- Plasma-derived (e.g., Humate-P, Wilate)
- Recombinant (e.g., vonicog alfa for select cases)
Dosing Strategy:
- Target VWF:RCo >50–100 IU/dL and FVIII >50 IU/dL
- Administer prior to surgery and continue postoperatively based on bleeding risk
Duration of Treatment:
- Minor procedures: 1–3 days
- Major surgeries: 5–10 days or longer as indicated
Antifibrinolytics (e.g., Tranexamic Acid, Aminocaproic Acid)
Used as adjuncts, especially in mucosal surgeries like dental or ENT procedures.
Route: Oral or IV
Caution: Avoid in patients with hematuria or thrombotic history
Surgical Procedure Categories and Recommended Prophylaxis
| Procedure Type | Risk Level | Recommended Approach |
|---|---|---|
| Dental extraction | Low | Desmopressin ± antifibrinolytics |
| Tonsillectomy, ENT | Moderate | DDAVP or factor replacement + antifibrinolytics |
| Orthopedic/Abdominal | High | VWF/FVIII concentrates pre-op & post-op |
| Neurosurgery/Cardiac | Very high | Intensive replacement therapy, ICU monitoring |
Postoperative Monitoring and Complication Management
Close monitoring is crucial in the postoperative period to detect delayed bleeding and thrombotic risks. Key parameters include:
- Plasma levels of VWF:RCo and FVIII:C
- Hemoglobin and hematocrit
- Wound site inspection
- Urine output and signs of hyponatremia (in DDAVP users)
Reassessment should guide tapering of therapy to avoid both bleeding and thromboembolic events.
Pediatric and Obstetric Considerations
Pediatric Surgery
Children with VWD undergoing surgery require weight-based dosing and specialized monitoring. Desmopressin is effective in most Type 1 cases but requires caution due to risk of hyponatremia.
Obstetric Interventions
Labor and delivery present significant hemorrhagic risk in women with VWD. Management includes:
- VWF and FVIII level assessment in third trimester
- Planning vaginal delivery when possible
- Factor replacement during labor and postpartum
Advancements and Future Directions
New generation recombinant VWF therapies offer precise dosing and pathogen safety. Gene therapy, though experimental, holds promise for long-term correction of VWF deficiency. Personalized prophylaxis guided by pharmacokinetic profiling will further optimize care.
Surgery-induced bleeding in von Willebrand’s disease requires individualized, risk-based preventive strategies. Through accurate diagnosis, timely prophylaxis using desmopressin or VWF/FVIII concentrates, and vigilant perioperative monitoring, we can ensure safe surgical outcomes. A multidisciplinary team approach involving hematologists, surgeons, anesthesiologists, and nursing staff is essential to execute this complex yet critical aspect of VWD management.

