Skin allografts remain indispensable in the management of extensive burns, chronic wounds, and certain reconstructive procedures. However, the immunogenic nature of skin, rich in antigen-presenting cells and vascularization, renders it particularly susceptible to acute and chronic rejection. Preventing skin allograft rejection requires a multifaceted approach that integrates immune modulation, histocompatibility matching, and graft monitoring.
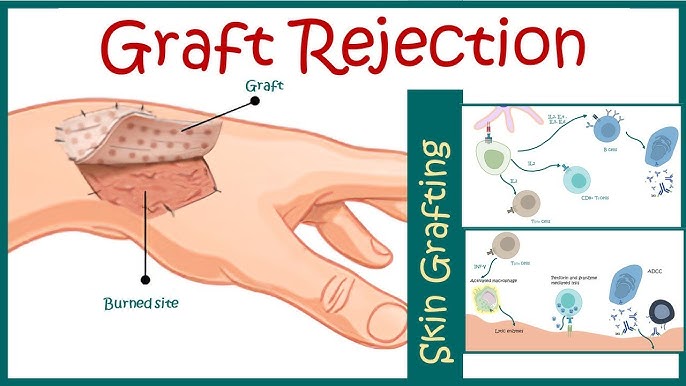
Immunopathogenesis of Skin Allograft Rejection
Allograft rejection occurs as a result of host immune system recognition of non-self antigens presented by the donor tissue. This initiates a cascade of cell-mediated and humoral immune responses.
Mechanisms Involved:
- Direct Allorecognition: Host T cells recognize donor MHC on graft-derived antigen-presenting cells (APCs).
- Indirect Allorecognition: Host APCs process donor antigens and present them to T cells.
- Effector Phase: Involves cytotoxic T lymphocytes (CTLs), natural killer (NK) cells, and proinflammatory cytokines such as IL-2, IFN-γ, and TNF-α.
- Humoral Response: Alloantibodies target graft vasculature, causing vascular thrombosis and ischemia.
The culmination of these processes leads to epidermal necrosis, inflammation, and graft failure.
HLA Matching and Histocompatibility in Graft Survival
The degree of HLA matching between donor and recipient is a critical determinant of graft longevity.
- HLA Class I and II typing minimizes immune activation.
- Full MHC compatibility is rarely possible in unrelated donors; however, partial matching significantly delays rejection.
- Use of tissue typing, panel-reactive antibody (PRA) testing, and crossmatching ensures compatibility and reduces preformed antibody-mediated rejection.
Immunosuppressive Regimens for Skin Allograft Acceptance
1. Calcineurin Inhibitors (CNI)
- Cyclosporine and Tacrolimus inhibit IL-2 transcription, thus suppressing T-cell activation.
- Effective in preventing acute rejection, though associated with nephrotoxicity and metabolic complications.
2. Antimetabolites
- Mycophenolate mofetil (MMF) and azathioprine impede lymphocyte proliferation.
- Used in combination with CNIs for synergistic suppression.
3. mTOR Inhibitors
- Sirolimus and everolimus inhibit T-cell proliferation and may promote regulatory T cell (Treg) survival.
4. Corticosteroids
- Administered for induction therapy and acute rejection episodes.
- Potent anti-inflammatory agents that reduce cytokine synthesis.
5. Biological Agents
- Anti-thymocyte globulin (ATG), basiliximab, and belatacept target specific immune pathways and are often used during induction or rejection episodes.
Tailored Immunosuppressive Strategy:
| Patient Risk | Recommended Regimen |
|---|---|
| Low Immunologic Risk | Tacrolimus + MMF + low-dose steroid |
| High Immunologic Risk | Tacrolimus + MMF + steroid + induction with ATG or basiliximab |
| Renal Concerns | Sirolimus-based CNI-free protocol |
Induction of Immune Tolerance in Skin Allografts
While systemic immunosuppression is effective, it carries long-term side effects. Inducing donor-specific immune tolerance provides a promising alternative for durable graft survival.
Strategies Include:
- Regulatory T Cells (Tregs): Adoptive transfer or expansion of Tregs to suppress effector T-cell responses.
- Mixed Chimerism: Bone marrow transplantation to induce host-donor tolerance.
- Costimulatory Blockade: Agents like CTLA4-Ig (abatacept) prevent T-cell activation without broad immunosuppression.
- Donor-Derived Exosomes or Tolerogenic Dendritic Cells: Modulate host immune response prior to grafting.
Experimental Approaches Under Investigation:
- CRISPR-modified allografts lacking MHC molecules.
- Nano-formulated immunosuppressants for site-specific drug delivery.
Graft Surveillance and Early Rejection Detection
Diagnostic Tools:
- Skin Biopsy: Gold standard for histologic confirmation; reveals perivascular lymphocytic infiltration, necrosis, and endothelial damage.
- Non-invasive Monitoring:
- Infrared thermography
- Laser Doppler flowmetry
- Molecular assays (e.g., donor-derived cell-free DNA)
- Serologic Tests:
- Monitoring DSAs (donor-specific antibodies) for early humoral rejection.
- Cytokine profiling to identify inflammatory flares.
Early detection facilitates timely escalation of immunosuppressive therapy, improving graft salvage rates.
Surgical and Technical Measures to Reduce Rejection Risk
While immunological control is paramount, surgical technique and wound environment also play a role in graft outcomes.
Best Practices:
- Use meshed grafts to enhance integration and drainage
- Ensure well-vascularized recipient beds
- Avoid tension and shearing at graft margins
- Employ negative pressure wound therapy (NPWT) to enhance adhesion and neovascularization
Role of Skin Substitutes and Bioengineered Allografts
Acellular dermal matrices (ADMs) and tissue-engineered skin constructs offer alternatives that reduce rejection risk by removing donor immune triggers.
Examples:
- Integra®: Bilayer matrix of bovine collagen and silicone
- AlloDerm®: Processed human dermis, devoid of viable cells
Though not a replacement for vascularized skin, these constructs serve as scaffolds for host tissue regeneration and reduce antigenicity.
Future Perspectives in Allograft Tolerance and Engineering
Emerging Frontiers:
- Gene Editing of Grafts: Removing MHC molecules via CRISPR/Cas9 to create “universal donor” skin
- Immune-Privileged Grafts: Engineered to express PD-L1 or HLA-G to suppress local immune responses
- Cellular Therapies: Infusion of tolerogenic macrophages, dendritic cells, or mesenchymal stem cells
- AI-Based Rejection Prediction Models: Leveraging machine learning to forecast rejection events based on clinical and molecular data
Summary of Key Points
- Skin allograft rejection is primarily driven by robust T-cell-mediated immune responses.
- Prevention requires HLA matching, immunosuppression, and early detection of rejection.
- Calcineurin inhibitors, antimetabolites, and biologics form the cornerstone of pharmacological prevention.
- Future therapies will focus on immune tolerance, genetic modification, and precision immunology.
Frequently Asked Questions:
Why are skin allografts prone to rejection?
Skin is highly immunogenic due to its abundant antigen-presenting cells and blood supply, making it a primary target for immune responses.
How can rejection be identified early?
Through clinical signs (redness, necrosis), biopsy, and emerging tools like serum biomarkers and cell-free donor DNA assays.
Are immunosuppressive drugs always necessary?
Yes, unless immune tolerance is induced. Chronic immunosuppression remains standard for long-term graft acceptance.
Can skin from unrelated donors be used safely?
With appropriate HLA matching, immunosuppression, and careful surveillance, unrelated donor grafts can be viable for temporary or permanent coverage.
What is the future of skin graft rejection prevention?
The focus is shifting toward personalized immune modulation, gene-edited universal grafts, and minimally toxic therapies.