Cytomegalovirus (CMV) is the most common opportunistic infection following cardiac transplantation. It is associated with direct effects such as CMV syndrome and tissue-invasive disease, as well as indirect consequences including graft rejection, allograft vasculopathy, and increased mortality. The prevention of CMV disease after cardiac transplantation is essential to ensure both graft and patient survival. A proactive, risk-based approach combining antiviral prophylaxis, preemptive therapy, and ongoing monitoring is the current standard of care.
Understanding CMV Risk in Cardiac Transplant Recipients
The risk of CMV disease post-cardiac transplant is primarily determined by the donor and recipient serostatus:
| Donor (D) | Recipient (R) | Risk Level |
|---|---|---|
| Positive (+) | Negative (−) | High Risk |
| Positive (+) | Positive (+) | Intermediate Risk |
| Negative (−) | Positive (+) | Intermediate Risk |
| Negative (−) | Negative (−) | Low Risk |
D+/R− patients are at highest risk due to primary CMV infection, which lacks pre-existing immunity and involves high-level viral replication.
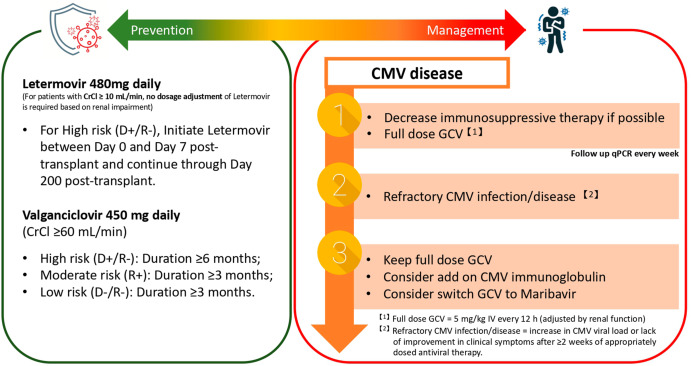
Antiviral Prophylaxis: Cornerstone of CMV Prevention
1. Valganciclovir
- First-line oral agent due to high bioavailability.
- Dose: 900 mg once daily, adjusted for renal function.
- Duration: 3 to 6 months in intermediate-risk; 6 to 12 months in high-risk (D+/R−) patients.
- Advantages: Reduces CMV syndrome, tissue-invasive disease, and CMV-related indirect effects.
2. Ganciclovir (IV)
- Indicated for patients unable to take oral medication or with severe gastrointestinal symptoms.
- Dose: 5 mg/kg IV every 12 hours during induction; then once daily for maintenance.
3. Letermovir
- Emerging alternative for patients intolerant to valganciclovir.
- Not yet approved for cardiac transplant patients but used in clinical trials and off-label settings.
Preemptive Therapy: Targeted Intervention Based on Viral Load
An alternative to universal prophylaxis in certain centers, preemptive therapy involves regular monitoring of CMV DNAemia using quantitative PCR and initiating treatment when viral replication is detected, prior to the onset of symptoms.
Monitoring Schedule:
- Frequency: Weekly for the first 3–6 months post-transplant
- Threshold: Viral load > 1000 IU/mL (center-specific)
- Treatment: Valganciclovir 900 mg BID until viral clearance
While cost-effective, this strategy requires robust infrastructure and immediate access to laboratory results and treatment initiation.
CMV Immune Monitoring and Diagnostic Tools
Quantitative PCR (qPCR)
- Gold standard for CMV detection
- Provides viral load trends, guides therapy, and detects breakthrough viremia
CMV-Specific Cell-Mediated Immunity Assays
- ELISPOT or QuantiFERON-CMV
- Predictive of protection against CMV disease
- Can inform prophylaxis duration and transition to monitoring phase
Adjunct Strategies for CMV Disease Prevention
Immunosuppression Modulation
- Calcineurin inhibitors (e.g., tacrolimus) may increase CMV replication risk
- Mycophenolate mofetil (MMF) use associated with higher CMV disease incidence
- Dose adjustment and balancing immunosuppression may reduce susceptibility
CMV Immunoglobulin (CMV-IVIG)
- Used in high-risk patients, especially with significant viremia or in combination with antivirals
- Provides passive immunity and immunomodulatory effects
- Not routinely recommended due to cost and lack of definitive evidence
Prevention of Late-Onset CMV Disease
Late-onset CMV disease often develops after cessation of prophylaxis, particularly in high-risk patients.
Strategies:
- Extended prophylaxis: 12 months in D+/R− individuals
- Secondary prophylaxis: Following treatment of CMV disease, for an additional 1–3 months
- Continued surveillance: Monthly CMV PCR for 3–6 months post-prophylaxis
Frequently Asked Questions
What is the best approach to prevent CMV disease after cardiac transplantation?
Valganciclovir prophylaxis tailored to the CMV risk profile remains the most effective strategy, particularly for high-risk (D+/R−) patients.
How long should CMV prophylaxis continue after a heart transplant?
- 6 to 12 months in high-risk patients
- 3 to 6 months in intermediate-risk individuals
- Consider extended prophylaxis in those receiving lymphocyte-depleting agents
What are the signs of CMV disease post-transplant?
- Fever, malaise, leukopenia (CMV syndrome)
- Gastrointestinal symptoms, hepatitis, pneumonitis (tissue-invasive disease)
- Laboratory-confirmed CMV DNAemia is a key marker
Can CMV infection lead to transplant rejection?
Yes, CMV increases the risk of acute and chronic rejection, contributing to cardiac allograft vasculopathy and poorer long-term outcomes.
Are there vaccines available for CMV prevention in transplant recipients?
No CMV vaccine is currently approved for clinical use, though several are in development.
The prevention of CMV disease after cardiac transplantation is a critical component of post-transplant care. A personalized approach based on CMV serostatus, use of effective antiviral prophylaxis, and rigorous virological monitoring ensures optimal outcomes. Advances in immunologic assays and the emergence of newer antiviral agents like letermovir may further refine prevention protocols, reducing CMV-associated morbidity and mortality in heart transplant recipients.