Chemotherapy-induced neurotoxicity, particularly peripheral neuropathy, is a frequent and often dose-limiting complication of cancer treatment. This form of neurotoxicity primarily affects sensory nerves and is commonly associated with agents such as platinum compounds, taxanes, vinca alkaloids, and proteasome inhibitors. Preventing neurotoxicity not only preserves functional integrity but also ensures uninterrupted and effective oncologic treatment.
Understanding Chemotherapy-Induced Neurotoxicity (CIPN)
Chemotherapy-induced peripheral neurotoxicity (CIPN) results from direct or indirect damage to the peripheral nervous system. Symptoms typically begin distally and symmetrically and may include tingling, numbness, burning, or pain. In severe cases, motor or autonomic nerve involvement can lead to functional impairment.
Key Chemotherapeutic Agents Linked to Neurotoxicity
| Drug Class | Examples | Neurotoxicity Features |
|---|---|---|
| Platinum compounds | Cisplatin, Oxaliplatin | Cumulative dose-related sensory neuropathy |
| Taxanes | Paclitaxel, Docetaxel | Acute and chronic peripheral neuropathy |
| Vinca alkaloids | Vincristine, Vinblastine | Dose-limiting motor neuropathy |
| Proteasome inhibitors | Bortezomib | Painful sensory neuropathy, dose-dependent |
| Thalidomide derivatives | Thalidomide, Lenalidomide | Length-dependent sensory impairment |
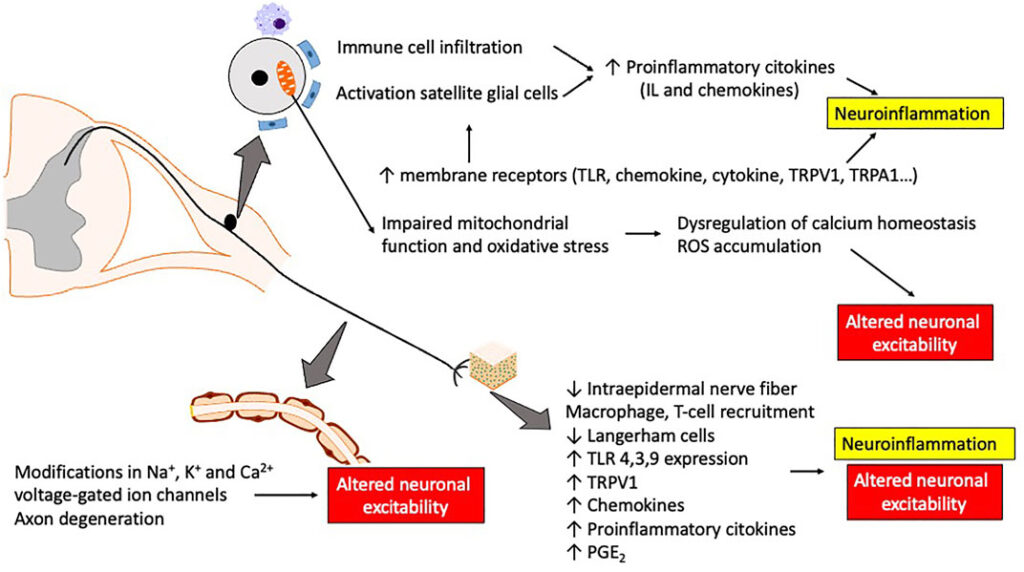
Pathophysiological Mechanisms Behind Neurotoxicity
Neurotoxic agents target various cellular components within neurons and supporting cells. Key mechanisms include:
- Axonal degeneration: Mitochondrial damage, oxidative stress
- Impaired axonal transport: Microtubule disruption (e.g., taxanes, vinca alkaloids)
- Dorsal root ganglion toxicity: DNA adduct formation (e.g., platinum agents)
- Neuroinflammation: Activation of glial cells and cytokine release
These mechanisms result in structural nerve damage and dysfunction, often irreversible if not addressed early.
Risk Factors for Chemotherapy-Induced Neurotoxicity
| Category | Risk Factors |
|---|---|
| Patient-related | Age >60, diabetes, alcohol use, genetic predisposition |
| Drug-related | High cumulative dose, infusion schedule, combination therapy |
| Disease-related | Pre-existing neuropathy, nutritional deficiencies |
Clinical Approaches for Prevention of Chemotherapy-Induced Neurotoxicity
1. Dose Optimization and Treatment Scheduling
Limiting the cumulative dose of neurotoxic agents is the most direct preventive approach. Strategies include:
- Dose reduction protocols
- Prolonged infusion durations to minimize peak plasma concentrations
- Rotational chemotherapy regimens to avoid continuous exposure
2. Pharmacologic Neuroprotective Agents
Several agents are under investigation or in use to mitigate neurotoxicity.
a. Duloxetine
- Mechanism: Serotonin-norepinephrine reuptake inhibition
- Indication: Shown to reduce painful CIPN symptoms
- Dose: 30–60 mg daily
- Evidence: Only agent with strong support in ASCO guidelines for symptom relief
b. Calcium and Magnesium Infusions
- Indication: Neuroprotection in oxaliplatin-induced neuropathy
- Protocol: 1g calcium gluconate and 1g magnesium sulfate pre/post infusion
- Controversy: Mixed evidence; some trials suggest no benefit
c. Glutathione
- Mechanism: Antioxidant, detoxifies platinum agents
- Use: Co-administered with cisplatin or oxaliplatin
- Evidence: Some clinical trials show reduced neurotoxicity
d. Vitamin E
- Antioxidant properties may protect neural tissue from oxidative damage
- Dose: 300–600 mg/day
- Results: Small studies show promise; not universally endorsed
e. Acetyl-L-Carnitine
- Mitochondrial cofactor
- Function: May support axonal regeneration
- Caution: Conflicting evidence on effectiveness
Monitoring and Early Detection of Neurotoxicity
Routine screening during chemotherapy enhances early detection and intervention.
Assessment Tools
- National Cancer Institute CTCAE grading
- Patient-reported outcome questionnaires
- Quantitative sensory testing (QST)
- Nerve conduction studies (in severe cases)
Monitoring Frequency
- Baseline neurofunction prior to therapy
- Assess at each cycle for high-risk drugs
- Post-treatment follow-up for delayed onset symptoms
Integrative and Non-Pharmacologic Approaches
Physical Therapy and Exercise
- Improves circulation and neuromuscular coordination
- Reduces fall risk and maintains mobility
Acupuncture
- May help in alleviating pain and dysesthesia
- Mixed results; beneficial in symptom control but not prevention
Cryotherapy
- Method: Cold gloves/socks during infusion
- Rationale: Vasoconstriction reduces drug delivery to extremities
- Evidence: Emerging data, especially for taxane-induced neuropathy
Best Practices for Neurotoxicity Prevention in Oncology
- Educate patients on early signs of neurotoxicity
- Implement standardized screening protocols
- Use validated patient-reported tools to track symptom evolution
- Document cumulative doses and adjust as needed
- Promote interdepartmental communication between oncologists, neurologists, and rehabilitation specialists
Frequently Asked Questions
Which chemotherapy drugs are most neurotoxic?
Cisplatin, oxaliplatin, paclitaxel, vincristine, and bortezomib are among the most commonly associated with neurotoxicity.
Can chemotherapy-induced neurotoxicity be reversed?
In many cases, it is partially reversible, but prolonged or severe cases may lead to permanent nerve damage.
How soon do symptoms appear?
Neurotoxicity may present within weeks of therapy initiation, but symptoms often accumulate over several cycles.
Are there FDA-approved preventive drugs?
There are no FDA-approved drugs specifically for prevention; however, duloxetine is used for symptom management post-onset.
What lifestyle changes help with prevention?
Maintaining optimal glycemic control, avoiding alcohol, engaging in light exercise, and managing vitamin deficiencies may reduce risk.
The prevention of chemotherapy-induced neurotoxicity demands a multifaceted approach, combining pharmacologic interventions, personalized dose management, physical rehabilitation, and vigilant monitoring. As neurotoxicity continues to be a challenge in oncology, a proactive stance not only minimizes long-term morbidity but also supports patients in completing their cancer treatments with preserved neurological function.