Chemotherapy-induced hemorrhagic cystitis (HC) is a serious urological complication predominantly associated with the use of alkylating agents such as cyclophosphamide and ifosfamide. It manifests as bladder mucosal bleeding, dysuria, hematuria, and potential long-term bladder dysfunction. Prevention is imperative to avoid treatment delays, hospitalizations, or severe morbidity. This comprehensive clinical guide outlines robust, evidence-backed strategies for preventing hemorrhagic cystitis in oncology patients receiving chemotherapeutic agents.
Pathophysiology of Chemotherapy-Induced Hemorrhagic Cystitis
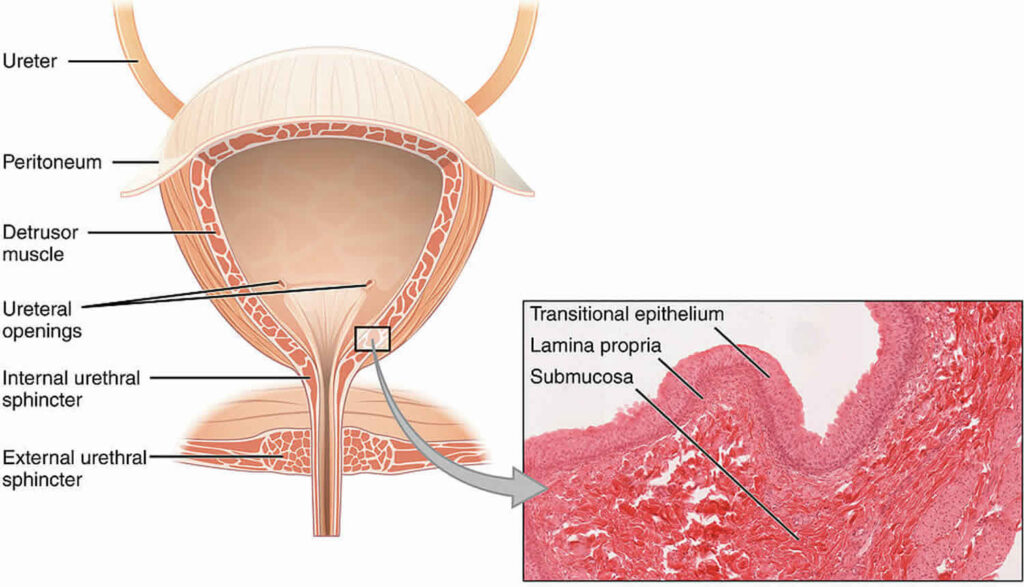
Hemorrhagic cystitis occurs when toxic metabolites, particularly acrolein, are excreted in urine and come into prolonged contact with the bladder urothelium. Acrolein, a byproduct of cyclophosphamide and ifosfamide metabolism, initiates urothelial inflammation, capillary damage, and hemorrhage.
Key Features of HC Pathogenesis
- Urothelial injury from acrolein accumulation
- Inflammatory cytokine release (e.g., IL-1, TNF-α)
- Capillary rupture leading to hematuria
- Fibrosis in chronic cases
Risk Factors for Developing Hemorrhagic Cystitis
| Risk Factor | Description |
|---|---|
| High-dose cyclophosphamide/ifosfamide | Dose-dependent toxicity |
| Inadequate hydration | Increases acrolein contact time in the bladder |
| Delayed MESNA administration | Incomplete uroprotection |
| Previous pelvic radiation | Compromised bladder vasculature |
| Age (pediatric or elderly) | Altered metabolism and excretion |
| Prolonged urinary retention | Promotes metabolite accumulation |
Core Strategies for the Prevention of Hemorrhagic Cystitis
1. MESNA Administration: The Standard of Uroprotection
2-Mercaptoethane Sulfonate Sodium (MESNA) is the cornerstone of preventive therapy. It binds to acrolein in the urinary tract, rendering it inert and non-toxic.
MESNA Protocol Guidelines
- IV or oral dosing: 20% of the cyclophosphamide/ifosfamide dose
- Dosing schedule: At 0, 4, and 8 hours post-chemotherapy
- Hydration requirement: Adequate fluid intake to ensure frequent urination
- Supplemental doses: In extended infusions, MESNA may be given continuously
MESNA must be timed meticulously to coincide with peak urinary acrolein levels.
2. Aggressive Intravenous Hydration
High-volume fluid therapy dilutes urinary metabolites and promotes frequent voiding, thereby minimizing urothelial contact.
- Fluid volume: 2–3 L/m²/day
- Initiate: 4–6 hours prior to chemotherapy
- Continue: 24 hours post-chemotherapy
- Use of diuretics: Optional (e.g., furosemide) to induce diuresis
3. Frequent Bladder Emptying
Urinary stasis enhances the urothelial exposure to acrolein. Scheduled voiding or catheterization ensures timely elimination.
- Bladder protocol: Every 2 hours during waking hours
- Nighttime strategy: Wake every 4 hours or use indwelling catheter
4. Bladder Irrigation in High-Risk Patients
For patients at high risk or undergoing hematopoietic stem cell transplantation, continuous bladder irrigation (CBI) may be employed.
- Infusion rate: 100–200 mL/hour of normal saline
- Duration: Throughout chemotherapy and 24–48 hours post-treatment
- Monitoring: Ensure patency and avoid bladder overdistension
5. Use of Alternative or Adjunctive Protective Agents
In cases of MESNA intolerance or as an added measure, certain agents may offer supplementary protection.
| Agent | Mechanism | Evidence Level |
|---|---|---|
| N-acetylcysteine (NAC) | Antioxidant, urothelial protection | Moderate |
| Prostaglandin analogs | Mucosal defense enhancer | Limited |
| Hyperhydration alone | Mechanical dilution of toxic metabolites | Effective but not standalone |
Special Considerations for Pediatric and Transplant Patients
Children and stem cell transplant recipients are particularly susceptible to severe HC due to higher chemotherapy intensity and immunosuppression.
Pediatric Protocol Adjustments
- Weight-adjusted MESNA and hydration dosing
- Avoid bladder overdistension
- Pain management with opioids and antispasmodics
Stem Cell Transplant Population
- Monitor for BK virus, which can exacerbate HC
- Employ CBI more liberally in conditioning regimens involving cyclophosphamide
Management of Breakthrough Hemorrhagic Cystitis
Despite preventive measures, breakthrough HC may occur and demands swift intervention.
Medical Management
- Antivirals (e.g., cidofovir) if BK virus implicated
- Anticholinergics for bladder spasms
- Pain control with opioids
Surgical Interventions
- Clot evacuation via cystoscopy
- Intravesical alum or formalin instillation
- Urinary diversion in refractory cases
Frequently Asked Questions
What is the primary cause of hemorrhagic cystitis during chemotherapy?
Acrolein, a metabolite of cyclophosphamide and ifosfamide, is the primary irritant responsible for bladder wall damage.
Is MESNA always required with cyclophosphamide?
MESNA is essential when cyclophosphamide or ifosfamide is used in high doses or prolonged regimens. Lower doses may not necessitate MESNA if aggressive hydration is maintained.
Can hemorrhagic cystitis be fatal?
Severe cases can lead to life-threatening hemorrhage, infection, or sepsis, especially in immunocompromised patients.
What are the early signs of hemorrhagic cystitis?
Symptoms include dysuria, urgency, hematuria, and lower abdominal discomfort. Prompt recognition is key to prevention of complications.
Is oral MESNA as effective as IV MESNA?
Yes, oral MESNA has comparable efficacy when dosed appropriately, though IV administration is preferred in cases of nausea or GI intolerance.
Preventing chemotherapy-induced hemorrhagic cystitis necessitates a proactive, multi-pronged strategy. By administering MESNA according to protocol, ensuring aggressive hydration, maintaining bladder hygiene, and monitoring high-risk patients, we can significantly mitigate the incidence and severity of this debilitating condition. A well-coordinated approach ensures oncologic efficacy is preserved without compromising urologic safety.