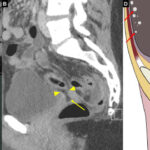
Post-transplant cytomegalovirus (CMV) infection is one of the most significant complications following solid organ and hematopoietic stem cell transplantation. This herpesvirus, typically latent in immunocompetent individuals, can reactivate under the immunosuppressive conditions post-transplantation, leading to severe and potentially life-threatening disease.
CMV infection contributes to both direct and indirect adverse outcomes, including organ dysfunction, increased risk of secondary infections, graft rejection, and reduced patient survival. A structured approach encompassing risk stratification, monitoring, prophylaxis, and timely treatment is essential in managing CMV post-transplant.
Etiology and Transmission of CMV in Transplant Recipients
CMV is a DNA virus belonging to the Herpesviridae family. It is transmitted via bodily fluids, with potential transmission modes in transplant settings including:
- Donor-derived infection: Transmitting CMV from a CMV-seropositive donor to a seronegative recipient (D+/R−) is the highest-risk scenario.
- Reactivation: CMV reactivation from the recipient’s latent infection under immunosuppression (R+).
- Community exposure: Less common but possible through external contact post-transplant.
Risk Stratification by Serostatus
Serologic matching of donor and recipient CMV status remains critical in predicting the likelihood of CMV infection and guiding preventive strategies.
| Donor/Recipient (D/R) Serostatus | Risk Level |
|---|---|
| D+/R− | High Risk |
| D+/R+ or D−/R+ | Intermediate Risk |
| D−/R− | Low Risk |
Patients undergoing lung, intestinal, or pancreas transplants are at higher risk compared to kidney or liver recipients due to the intensity of immunosuppression and tissue CMV tropism.
Clinical Manifestations of CMV Disease
CMV infection encompasses a spectrum from asymptomatic viremia to end-organ disease:
1. CMV Syndrome
Characterized by systemic symptoms such as:
- Fever
- Malaise
- Leukopenia
- Thrombocytopenia
- Elevated liver enzymes
2. Tissue-Invasive CMV Disease
Includes:
- CMV colitis: Diarrhea, abdominal pain, GI bleeding
- CMV pneumonitis: Cough, dyspnea, hypoxia
- CMV hepatitis: Elevated transaminases, hepatomegaly
- CMV retinitis (mainly in stem cell recipients): Blurred vision, floaters, vision loss
CMV disease can exacerbate rejection episodes or worsen graft function, making early intervention crucial.
Diagnostic Approaches
1. CMV DNA PCR (Quantitative Nucleic Acid Testing)
- Most sensitive and specific test for CMV viremia
- Allows for viral load monitoring and preemptive therapy
2. CMV pp65 Antigenemia
- Detects viral proteins in leukocytes
- Less commonly used due to lower sensitivity in neutropenic patients
3. Tissue Biopsy and Histopathology
- Required to confirm invasive CMV disease
- Look for inclusion bodies (“owl’s eye” appearance) and immunohistochemistry for CMV antigens
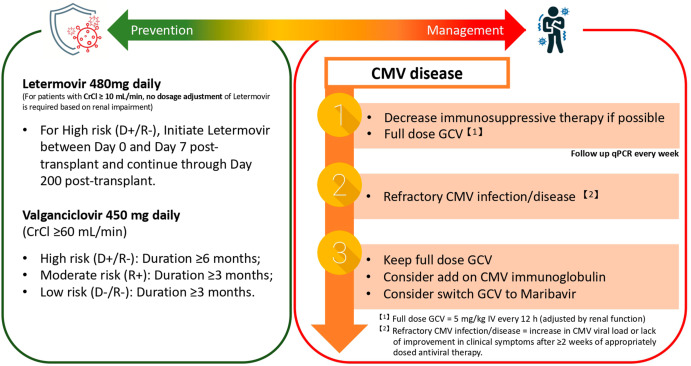
Antiviral Treatment Strategies
1. First-Line Therapy
- Intravenous ganciclovir (5 mg/kg every 12 hours) for severe or invasive disease
- Oral valganciclovir (900 mg twice daily) for mild to moderate cases
Duration typically spans 2 to 4 weeks, with continuation until undetectable viral load is sustained.
2. Alternative Agents
- Foscarnet: Used in cases of ganciclovir resistance or toxicity
- Cidofovir: Reserved for refractory infections due to nephrotoxicity
3. Adjunctive Therapy
- Immunoglobulin therapy: Considered in CMV pneumonitis
- Reduction of immunosuppression: Essential to aid immune control of CMV
CMV Prophylaxis and Preemptive Strategies
Preventing CMV post-transplant involves two primary strategies:
1. Universal Prophylaxis
- All at-risk patients receive antivirals immediately post-transplant for a defined duration
- Regimens:
- Valganciclovir 900 mg daily for 3–6 months, depending on organ type and risk
2. Preemptive Therapy
- Regular monitoring of CMV DNA levels
- Antivirals initiated at defined viral load thresholds before symptoms arise
- Common in intermediate-risk or resource-limited settings
Each strategy has pros and cons, and the choice depends on institutional protocols and patient-specific factors.
Monitoring and Resistance Considerations
Viral Load Monitoring
- Weekly during early post-transplant period
- Adjust antiviral duration based on CMV DNA trends
Drug Resistance
- Suspect in patients with rising viral load despite therapy
- Confirm via genotypic resistance testing
- Switch to foscarnet or combination therapy as needed
Long-Term Complications and Indirect Effects
Even after clearance, CMV can have lasting consequences:
- Chronic allograft dysfunction
- Increased susceptibility to bacterial and fungal infections
- Enhanced risk of Epstein-Barr virus reactivation
- Post-transplant lymphoproliferative disorder (PTLD), especially in EBV-naïve patients
Future Directions and Vaccine Research
Although no licensed vaccine currently exists, CMV vaccine candidates are under development. Promising modalities include:
- Live-attenuated vaccines
- mRNA-based platforms
- CMV-specific T-cell immunotherapy for high-risk or resistant cases
Advances in CMV immunology and vaccine research aim to reduce dependency on prolonged antiviral prophylaxis.
Post-transplant cytomegalovirus infection poses significant challenges in transplant medicine. By integrating robust risk stratification, preemptive monitoring, timely therapeutic intervention, and evidence-based prophylactic protocols, we can mitigate CMV’s impact on patient morbidity and graft survival. Continued advancements in diagnostic technology, antiviral agents, and vaccine development hold the key to a future with fewer CMV-related complications in transplant recipients.