Philadelphia chromosome-positive chronic myelocytic leukemia (Ph+ CML) is a hematopoietic stem cell disorder characterized by unregulated myeloid cell proliferation, resulting from the formation of the BCR-ABL1 fusion gene. This molecular abnormality is the hallmark of CML and plays a central role in its pathogenesis, diagnosis, and treatment.
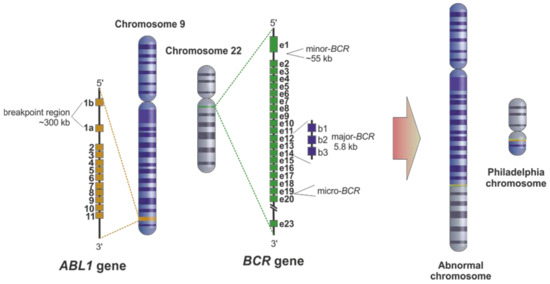
Pathogenesis of Ph+ CML: The Role of BCR-ABL1 Fusion
The Philadelphia chromosome arises from a reciprocal translocation between chromosomes 9 and 22, denoted as t(9;22)(q34;q11.2). This results in the BCR-ABL1 fusion gene, which encodes a constitutively active tyrosine kinase enzyme, leading to continuous signaling for cell proliferation, reduced apoptosis, and genomic instability.
Molecular Mechanism
- BCR gene (chromosome 22) joins with ABL1 gene (chromosome 9)
- The resultant protein activates several downstream signaling pathways: RAS, JAK-STAT, PI3K/AKT
- Drives chronic expansion of granulocytic lineage
Clinical Phases of Chronic Myelocytic Leukemia
Ph+ CML evolves through three clinical phases, each with distinct prognostic and therapeutic implications.
1. Chronic Phase (CP)
- Most patients (~90%) are diagnosed in this phase
- Symptoms often mild: fatigue, splenomegaly, leukocytosis
- Responds well to tyrosine kinase inhibitors (TKIs)
2. Accelerated Phase (AP)
- Defined by increased blasts (10–19%), basophils ≥20%, or progressive cytogenetic abnormalities
- Indicates disease progression and resistance
3. Blast Crisis (BC)
- ≥20% blasts in bone marrow or peripheral blood
- Transforms into acute leukemia (usually myeloid or lymphoid)
- Poor prognosis requiring aggressive therapy
Diagnostic Criteria and Workup
Laboratory Investigations
- Complete Blood Count (CBC): Leukocytosis, basophilia, eosinophilia
- Peripheral blood smear: Shows myelocytes, metamyelocytes, and other immature granulocytes
- Bone marrow aspirate: Hypercellular with granulocytic predominance
Cytogenetic and Molecular Testing
- FISH (Fluorescence In Situ Hybridization): Detects Philadelphia chromosome
- RT-PCR: Quantifies BCR-ABL1 transcripts
- Karyotyping: Essential for baseline risk assessment
Tyrosine Kinase Inhibitor (TKI) Therapy: Mainstay of Treatment
The advent of TKIs has transformed the prognosis of Ph+ CML, turning it into a manageable chronic disease.
First-Generation TKI
- Imatinib (Gleevec): Introduced in early 2000s; revolutionized treatment by targeting BCR-ABL1 selectively
Second-Generation TKIs
- Dasatinib: Effective against many imatinib-resistant mutations
- Nilotinib: Offers faster and deeper molecular responses
- Bosutinib: Effective with fewer off-target effects
Third-Generation TKI
- Ponatinib: Designed to overcome T315I mutation; used in resistant or advanced cases
Monitoring Response and Molecular Milestones
Milestones Defined by ELN Guidelines
| Timepoint | Target Response |
|---|---|
| 3 Months | BCR-ABL1 ≤10% (early molecular response) |
| 6 Months | BCR-ABL1 ≤1% |
| 12 Months | BCR-ABL1 ≤0.1% (major molecular response) |
| Ongoing | MR4/MR4.5 (deep molecular remission) |
Failure to achieve these targets necessitates re-evaluation of therapy and mutational analysis.
Risk Scoring Systems for Prognosis
Several scoring models aid in baseline risk assessment and treatment decisions:
- Sokal Score
- Hasford (Euro) Score
- EUTOS Score
- ELTS Score (most current)
Each considers age, spleen size, platelet count, and blast percentage.
Resistance to TKI Therapy: Mechanisms and Management
Causes of Resistance
- BCR-ABL1 mutations (e.g., T315I, Y253H)
- Pharmacokinetic issues: poor absorption, drug interactions
- Disease-related: clonal evolution, additional cytogenetic abnormalities
Management Strategies
- Mutation analysis to identify resistant clones
- Switch to second- or third-generation TKI based on mutation profile
- Allogeneic stem cell transplant (allo-SCT) in advanced/refractory cases
Treatment-Free Remission (TFR): A New Frontier
TFR refers to sustained molecular remission after TKI discontinuation in select patients.
Criteria for TFR Eligibility
- ≥3 years of sustained deep molecular response (MR4 or MR4.5)
- Consistent monitoring capability (monthly PCR)
- Absence of disease progression or resistance
Approximately 40–60% of eligible patients can maintain remission without ongoing therapy.
Allogeneic Stem Cell Transplantation in CML
Although no longer first-line, allo-SCT remains a potential curative option for:
- Blast phase disease
- TKI failure with resistance mutations
- Young patients with matched donors and high-risk features
Emerging Therapies and Future Directions
- Asciminib: STAMP inhibitor (Specifically Targets ABL Myristoyl Pocket); novel mechanism and effective in TKI-resistant CML
- Combination therapies: TKIs + immune modulation under investigation
- Gene editing: CRISPR/Cas9-based approaches to eliminate BCR-ABL1
Comparison of Key TKIs in Ph+ CML
| TKI | Generation | Mutation Coverage | Common Side Effects | Used In |
|---|---|---|---|---|
| Imatinib | 1st | Limited | Edema, GI upset | Frontline, chronic phase |
| Dasatinib | 2nd | Broad (except T315I) | Pleural effusion, cytopenia | Frontline, resistance to imatinib |
| Nilotinib | 2nd | Broad (except T315I) | QT prolongation, rash | Frontline or 2nd line |
| Bosutinib | 2nd | Broad (except T315I) | Diarrhea, liver enzymes | Intolerant/resistant patients |
| Ponatinib | 3rd | T315I and all others | Arterial events, hypertension | Advanced phase, T315I mutation |
Philadelphia chromosome-positive chronic myelocytic leukemia is a well-characterized myeloproliferative disorder with a clearly defined molecular driver: BCR-ABL1. The targeted therapy revolution, led by tyrosine kinase inhibitors, has drastically transformed CML into a manageable, and in some cases, potentially curable disease. With continued advancements in molecular monitoring, precision medicine, and novel therapeutic agents, the outlook for patients with Ph+ CML continues to improve. Strategic monitoring and timely interventions remain essential to optimize outcomes across all phases of the disease.