Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) represents a high-risk subtype of ALL, characterized by the presence of the BCR-ABL1 fusion gene resulting from a translocation between chromosomes 9 and 22. This genetic anomaly drives aggressive leukemic proliferation and has historically been associated with poor prognosis. However, with the advent of tyrosine kinase inhibitors (TKIs) and precision medicine, outcomes have significantly improved.
Pathogenesis and Molecular Characteristics of Ph+ ALL
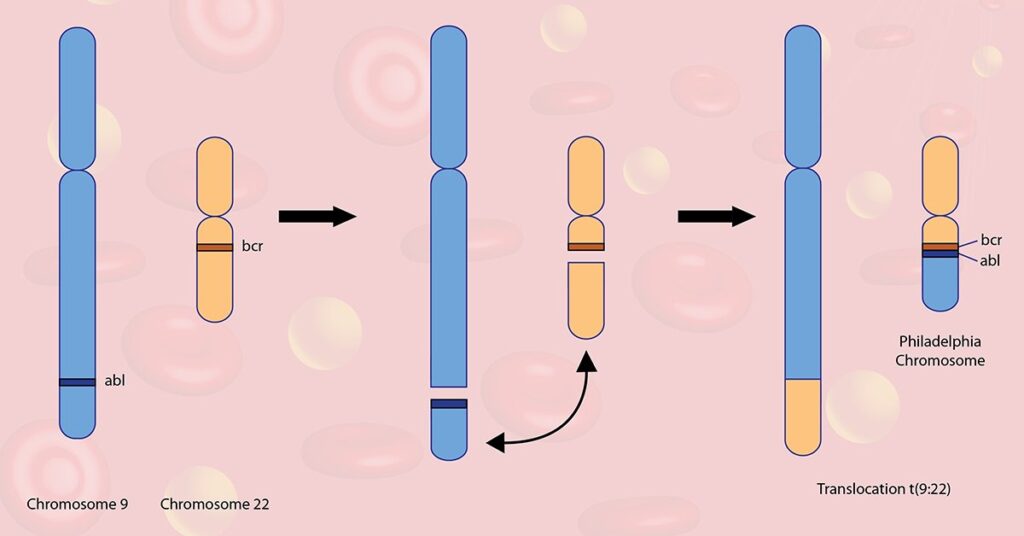
Ph+ ALL results from the t(9;22)(q34;q11.2) translocation, creating the Philadelphia chromosome, which fuses the BCR gene on chromosome 22 with the ABL1 gene on chromosome 9. This fusion results in the constitutive activation of a tyrosine kinase, promoting unregulated cell division and impaired apoptosis.
Key Molecular Features
- BCR-ABL1 fusion: Central oncogenic driver
- p190 BCR-ABL isoform: Predominant in Ph+ ALL, compared to p210 in CML
- Additional mutations: IKZF1, PAX5, CDKN2A, and others often coexist, worsening prognosis
Epidemiology and Risk Stratification
- Incidence: Occurs in 20–30% of adult ALL and ~5% of pediatric ALL cases
- Age correlation: More prevalent with increasing age
- Prognosis: Historically poor, but significantly improved with TKIs and allogeneic stem cell transplantation (allo-SCT)
Clinical Presentation and Diagnosis
Symptoms
- Fatigue, pallor, easy bruising
- Fever, infections due to neutropenia
- Bone pain, lymphadenopathy, hepatosplenomegaly
Diagnostic Workup
- Peripheral blood smear and CBC: Reveals leukocytosis, anemia, thrombocytopenia
- Bone marrow aspiration: Confirms >25% lymphoblasts
- Cytogenetics and FISH: Identify t(9;22)
- RT-PCR or quantitative PCR: Detects and quantifies BCR-ABL1 transcripts for monitoring
Treatment of Philadelphia Chromosome-Positive ALL
Initial Induction Therapy
Historically, induction involved multi-agent chemotherapy, but current protocols integrate TKIs from the start.
Common Induction Regimens:
- Hyper-CVAD + TKI (e.g., imatinib or dasatinib)
- CALGB 10403 (pediatric-inspired) + TKI
- Mini-Hyper-CVD + dasatinib or ponatinib (in elderly patients)
Tyrosine Kinase Inhibitors in Ph+ ALL
TKIs target the BCR-ABL1 fusion protein and are pivotal in achieving molecular remission.
First-Generation TKI
- Imatinib: First TKI used; improved survival when combined with chemotherapy.
Second-Generation TKIs
- Dasatinib: Crosses blood-brain barrier (BBB), effective against CNS disease
- Nilotinib: Potent TKI with favorable toxicity in select cases
Third-Generation TKI
- Ponatinib: Effective against T315I mutation; used in relapsed/refractory or mutation-positive cases
TKI Therapy Considerations
- Monitoring BCR-ABL1 levels: Quantitative PCR every 3 months
- Mutation testing: Guides switch to more potent TKIs if resistance emerges
Consolidation and Maintenance Therapy
Allogeneic Stem Cell Transplantation (allo-SCT)
- Recommended for eligible patients in first complete remission (CR1), particularly those with suboptimal molecular response
- Offers potential for long-term cure
Maintenance Therapy
- TKI monotherapy or TKI + low-intensity chemotherapy (e.g., methotrexate, mercaptopurine)
- Continued molecular monitoring essential
Central Nervous System (CNS) Prophylaxis
- CNS relapse risk necessitates intrathecal chemotherapy with methotrexate or cytarabine
- Dasatinib offers added protection due to BBB penetration
Treatment in Relapsed/Refractory Ph+ ALL
Salvage Options
- Blinatumomab + TKI: Bispecific T-cell engager showing efficacy in relapsed disease
- Inotuzumab ozogamicin: Anti-CD22 antibody-drug conjugate for CD22+ ALL
- Ponatinib + chemotherapy: Particularly for T315I mutation
- CAR T-cell therapy: Emerging approach in relapsed cases
- Second allo-SCT: Considered in select relapsed patients
Prognostic Factors in Philadelphia Chromosome-Positive ALL
| Factor | Prognostic Implication |
|---|---|
| Age < 35 years | Favorable |
| Early molecular response | Favorable |
| Presence of T315I mutation | Poor prognosis |
| Lack of MRD negativity | Associated with relapse |
| Donor availability for SCT | Influences long-term outcome |
Minimal Residual Disease (MRD) in Treatment Monitoring
MRD Significance
- MRD is the strongest predictor of relapse
- Assessed using qPCR for BCR-ABL1 or flow cytometry
- MRD negativity after induction and consolidation correlates with superior overall survival
Differences Between Ph+ ALL and CML in Lymphoid Blast Crisis
| Feature | Ph+ ALL | CML Lymphoid Blast Crisis |
|---|---|---|
| BCR-ABL Isoform | p190 (mostly) | p210 (mostly) |
| Disease History | De novo | Preceded by chronic phase |
| Additional Mutations | IKZF1, CDKN2A, etc. | ASXL1, RUNX1 |
| Treatment Approach | TKI + chemotherapy + SCT | More resistant, poor prognosis |
Future Directions in Ph+ ALL Management
Novel Combinations
- Blinatumomab + TKIs in frontline setting under clinical evaluation
- Chemotherapy-free regimens with TKI + immunotherapy may redefine standard care
CAR T-Cell Therapy
- Anti-CD19 CAR-T cells have shown promising response rates in relapsed/refractory Ph+ ALL
Precision Medicine
- Genomic sequencing to personalize TKI selection
- mRNA-based strategies for MRD detection
Philadelphia chromosome-positive acute lymphoblastic leukemia remains a complex, aggressive leukemia subtype. The integration of TKIs into multi-agent regimens has revolutionized prognosis, transforming a once-fatal diagnosis into a potentially curable disease. Close molecular monitoring, timely intervention with stem cell transplantation, and novel targeted therapies continue to drive improved outcomes. Advances in precision oncology hold the promise of individualized, chemotherapy-free regimens in the near future.