Peripheral mobilization of hematopoietic stem cells (HSCs) refers to the clinical process of inducing the movement of stem cells from the bone marrow into the peripheral blood, allowing for their subsequent collection via apheresis. This technique has revolutionized hematopoietic stem cell transplantation (HSCT), offering a less invasive and more efficient alternative to direct bone marrow harvest.
Peripheral blood stem cell (PBSC) mobilization enables high-yield collection of CD34+ progenitor cells critical for successful engraftment, particularly in patients undergoing autologous or allogeneic stem cell transplantation for hematologic malignancies and other disorders.
Biological Basis of HSC Mobilization
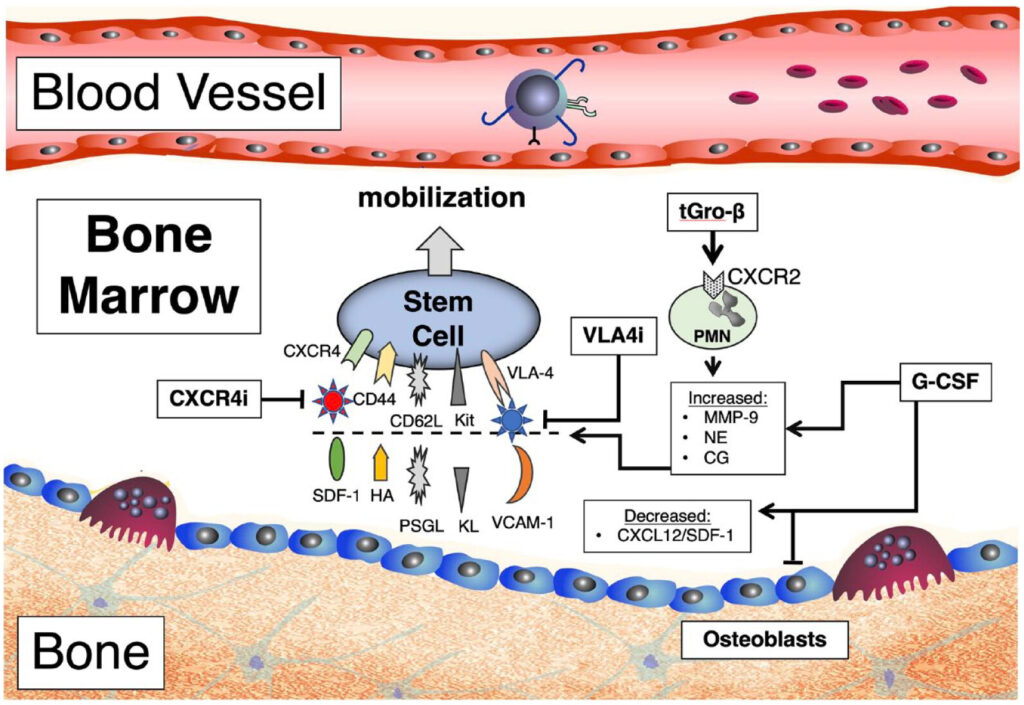
The mobilization of hematopoietic stem cells is regulated through the disruption of molecular interactions that normally anchor stem cells within the bone marrow microenvironment.
Key Mechanisms:
- SDF-1/CXCR4 Axis: Stromal cell-derived factor-1 (SDF-1) binds CXCR4 on HSCs, retaining them in bone marrow. Mobilizing agents disrupt this interaction.
- VCAM-1/VLA-4 Pathway: HSC adhesion to stromal cells is also mediated by vascular cell adhesion molecule-1 (VCAM-1); its inhibition promotes HSC release.
- Matrix Metalloproteinases (MMPs): Enzymes like MMP-9 degrade extracellular matrix components, further loosening HSCs from their niches.
Mobilizing Agents and Strategies
Peripheral stem cell mobilization can be achieved using various pharmacologic agents and regimens tailored to patient-specific variables.
1. Granulocyte Colony-Stimulating Factor (G-CSF)
The standard agent used for HSC mobilization:
- Administered subcutaneously, typically 10 μg/kg/day for 4–5 days
- Increases neutrophil counts and indirectly promotes stem cell egress
- Used alone or in combination with other agents
2. Plerixafor (AMD3100)
A CXCR4 antagonist approved for poor mobilizers:
- Disrupts SDF-1/CXCR4 binding
- Administered with G-CSF for synergistic effect
- Enhances CD34+ cell yield, especially in lymphoma and multiple myeloma patients
3. Chemotherapy-Based Mobilization (Chemomobilization)
Uses cytotoxic agents (e.g., cyclophosphamide) followed by G-CSF:
- Utilized in patients with prior poor mobilization
- Promotes both tumor cytoreduction and stem cell release
- Requires more intensive monitoring and support
4. Experimental Agents and Approaches
Emerging strategies include:
- VLA-4 inhibitors (e.g., BIO5192)
- S1P receptor modulators
- Parathyroid hormone (PTH) analogs
These are under investigation for improving mobilization efficiency and minimizing side effects.
Mobilization Timeline and Process Overview
The clinical process follows a structured timeline from mobilization to collection:
- Target CD34+ cell dose: >2 × 10⁶ cells/kg (autologous), >4 × 10⁶ cells/kg (allogeneic)
- Collection window: Usually spans 1–3 days depending on mobilization efficacy
Predictors of Mobilization Success
Certain patient-related and treatment-related factors influence mobilization outcomes:
| Factor | Impact on Mobilization |
|---|---|
| Prior chemotherapy or radiation | Decreases stem cell yield |
| Age >60 years | Associated with poorer response |
| Bone marrow involvement | Lowers CD34+ cell count |
| Disease type (e.g., myeloma) | Variable mobilization potential |
| Mobilization agent used | Plerixafor improves response |
Routine pre-mobilization assessments, including baseline complete blood counts and marrow imaging, guide regimen selection and predict outcomes.
Apheresis and CD34+ Cell Collection
Apheresis is the extracorporeal process used to collect mobilized HSCs from peripheral blood:
- Procedure: Blood is drawn, stem cells separated, and remaining components returned to the patient.
- Duration: 3–6 hours per session
- Monitoring: Peripheral CD34+ count dictates timing and number of sessions
- Side effects: Mild, including citrate-related hypocalcemia, nausea, and fatigue
Advanced collection systems ensure high efficiency and low contamination with red or white blood cells.
Indications for PBSC Mobilization
Peripheral stem cell mobilization is a cornerstone in the management of various hematologic and genetic conditions:
Autologous Transplantation:
- Multiple myeloma
- Non-Hodgkin lymphoma
- Hodgkin lymphoma
- Amyloidosis
Allogeneic Transplantation:
- Acute leukemias
- Myelodysplastic syndromes
- Aplastic anemia
- Inherited marrow failure syndromes
PBSCs are increasingly preferred over bone marrow for allogeneic transplants due to faster engraftment and reduced relapse in certain settings.
Risks and Limitations
Despite its advantages, mobilization is not without risks:
- G-CSF-related side effects: Bone pain, headache, splenic enlargement (rare)
- Mobilization failure: Occurs in ~10–15% of patients; salvage with plerixafor
- Disease contamination: Higher risk in autologous collections; careful screening required
- Cost and logistics: Plerixafor is expensive; multiple hospital visits required
Close monitoring and individualized strategies mitigate these challenges.
Advances and Future Directions
Research continues to refine mobilization protocols:
- Biomarker-based prediction models for mobilization success
- Gene expression profiling to identify poor mobilizers
- Novel small molecule inhibitors for adhesion pathways
- Automation and AI integration in apheresis monitoring
Personalized medicine approaches aim to match the optimal mobilization strategy to each patient’s genetic and clinical profile.
Peripheral mobilization of hematopoietic stem cells has transformed stem cell transplantation by enabling efficient, high-yield collection of CD34+ cells through minimally invasive means. Through the use of pharmacologic agents such as G-CSF and plerixafor, as well as evolving mobilization regimens, clinicians can effectively prepare patients for both autologous and allogeneic transplantation. With ongoing advances in stem cell biology and mobilization techniques, outcomes are expected to improve further, ensuring broader applicability and better patient care in hematologic diseases.