Peptococcus peritonitis refers to a serious intra-abdominal infection caused by Peptococcus, a genus of anaerobic, gram-positive cocci. While normally part of the mucosal flora of the skin, oral cavity, gastrointestinal, and genitourinary tracts, Peptococcus can become pathogenic when introduced into the normally sterile peritoneal cavity. This condition often develops as part of a polymicrobial infection and is associated with significant morbidity, particularly in immunocompromised individuals or patients with underlying gastrointestinal pathology.
Pathophysiology: How Peptococcus Causes Peritonitis
Peritonitis results from bacterial contamination of the peritoneal cavity, either via direct perforation, hematogenous spread, or translocation from adjacent inflamed organs. In the case of Peptococcus, its anaerobic nature thrives in low-oxygen environments, allowing it to proliferate and contribute to abscess formation and systemic inflammatory responses.
Etiology and Risk Factors of Peptococcus Peritonitis
Common Routes of Infection:
- Ruptured appendicitis or diverticulitis
- Bowel perforation from trauma or surgery
- Complications from dialysis (peritoneal catheter contamination)
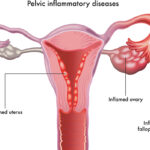
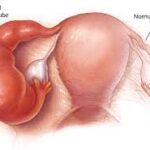
- Gynecological sources (e.g., pelvic inflammatory disease or tubo-ovarian abscess)
- Postoperative infections following abdominal surgery
Key Risk Factors:
- Immunosuppression (e.g., chemotherapy, HIV)
- Poorly controlled diabetes mellitus
- Chronic kidney disease requiring peritoneal dialysis
- Inflammatory bowel disease
- Prolonged antibiotic use leading to microbiota imbalance
- Malignancy with intra-abdominal spread
Clinical Presentation and Symptoms
Patients with Peptococcus peritonitis typically present with a constellation of systemic and localized abdominal symptoms, often indistinguishable from other causes of peritonitis. However, the slow-growing, anaerobic nature of the organism can result in delayed onset or subtle progression.
Signs and Symptoms:
- Generalized or localized abdominal pain
- Abdominal distension
- Fever, chills, and tachycardia
- Nausea and vomiting
- Guarding and rebound tenderness
- Decreased bowel sounds or paralytic ileus
- Hypotension in severe or septic presentations
Diagnostic Evaluation of Anaerobic Peritonitis
Accurate and timely diagnosis of Peptococcus peritonitis is crucial to prevent complications such as abscesses, sepsis, or multi-organ failure.
Laboratory Investigations:
- Complete blood count (CBC): Leukocytosis with left shift
- C-reactive protein (CRP), ESR, and procalcitonin: Elevated
- Blood cultures: May reveal anaerobic bacteremia
- Ascitic fluid analysis: High neutrophil count, low glucose, high protein
- Gram stain and anaerobic culture of peritoneal fluid: Identification of Peptococcus
Imaging Studies:
- Abdominal CT scan with contrast:
- Locates fluid collections or abscesses
- Identifies source of infection (e.g., perforation, inflamed bowel)
- Ultrasound: Useful in dialysis-associated or gynecologic-associated infections
Microbiological Features of Peptococcus
Peptococcus is characterized by the following:
- Gram-positive anaerobic cocci
- Found in pairs, chains, or clusters
- Non-spore forming and non-motile
- Grows in anaerobic culture media within 48–72 hours
- Frequently isolated in polymicrobial cultures, particularly with Bacteroides, Fusobacterium, or Enterobacteriaceae
Due to its slow growth and oxygen sensitivity, anaerobic culturing techniques must be employed immediately after sample collection.
Management and Treatment Strategies
Effective management of Peptococcus peritonitis relies on a combination of antibiotic therapy, source control, and supportive care. Treatment must address both the anaerobic component and any co-infecting aerobic organisms.
Empirical Antibiotic Therapy:
Empiric regimens should always include anaerobic coverage. Upon confirmation of Peptococcus, therapy should be adjusted based on sensitivities.
First-Line Options:
- Piperacillin-tazobactam 4.5 g IV q8h
- Meropenem 1 g IV q8h (especially for polymicrobial resistance)
- Metronidazole 500 mg IV/PO q8h in combination with:
- Ceftriaxone 2 g IV daily, or
- Ciprofloxacin 400 mg IV q12h
Duration of Therapy:
- Uncomplicated peritonitis: 7–10 days
- With abscess or severe sepsis: 14–21 days depending on clinical resolution
Surgical or Interventional Source Control:
- Abscess drainage: Percutaneous or surgical
- Laparotomy or laparoscopy: To repair perforated viscera or resect necrotic tissue
- Dialysis catheter removal or replacement in PD-associated peritonitis
Complications and Prognosis
Anaerobic peritonitis caused by Peptococcus can escalate to life-threatening conditions if left untreated. The following complications are commonly observed:
Major Complications:
- Intra-abdominal abscess formation
- Bowel obstruction from adhesions
- Septic shock
- Multi-organ dysfunction syndrome (MODS)
- Persistent or relapsing peritonitis in dialysis patients
- High mortality rate in immunocompromised patients
Prognosis significantly improves with early diagnosis, adequate source control, and effective anaerobic antibiotic therapy.
Prevention and Infection Control
Preventing Peptococcus peritonitis involves minimizing the risk of bacterial translocation, improving aseptic practices, and managing comorbidities effectively.
Prevention Strategies:
- Aseptic technique during peritoneal dialysis exchanges
- Timely management of intra-abdominal pathology (e.g., appendicitis, diverticulitis)
- Postoperative surveillance for infection signs
- Judicious antibiotic use to avoid dysbiosis
- Patient education on catheter hygiene in home-based dialysis programs
Peptococcus peritonitis represents a clinically significant anaerobic infection of the peritoneal cavity, often occurring in polymicrobial contexts and linked to gastrointestinal or genitourinary pathology. Its insidious onset, diagnostic complexity, and potential for serious complications necessitate a vigilant clinical approach. By integrating advanced diagnostic tools, appropriate empirical therapy with anaerobic coverage, and timely surgical intervention, we can achieve optimal outcomes in managing this challenging condition.