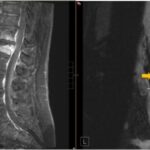
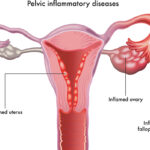
Peptococcus pelvic cellulitis is a severe anaerobic infection involving the soft tissues of the female pelvis, caused by Peptococcus species—gram-positive, non-motile, anaerobic cocci that are part of the normal vaginal and gastrointestinal flora. While typically non-pathogenic in a healthy host, these organisms can become opportunistic pathogens in the presence of mucosal disruption, surgical procedures, or immunosuppression, leading to deep-seated infections such as pelvic cellulitis and abscess formation.
Pathogenesis and Infection Route
Peptococcus species thrive in low-oxygen environments and invade pelvic tissues through endogenous translocation or direct introduction during gynecological interventions.
Common sources of mucosal disruption include postpartum trauma, pelvic surgery, intrauterine device (IUD) insertion, or pelvic inflammatory disease (PID).
Epidemiology and Risk Factors
Peptococcus pelvic infections are infrequent but clinically significant, often underdiagnosed due to the fastidious nature of anaerobes in culture.
Risk Factors:
- Postpartum or post-abortion infections
- Pelvic or abdominal surgery
- IUD usage
- Bacterial vaginosis or cervicitis
- Advanced age or diabetes mellitus
- Immunosuppression (HIV, corticosteroid therapy)
- Pelvic malignancies or radiation therapy
Clinical Manifestations of Pelvic Cellulitis Due to Peptococcus
The presentation is often subacute, with non-specific symptoms that mimic other pelvic or abdominal pathologies. Prompt identification is critical to prevent systemic progression.
Key Symptoms:
- Persistent lower abdominal or pelvic pain
- Pelvic tenderness with or without rebound
- Fever and chills
- Foul-smelling vaginal discharge
- Malaise and fatigue
- Dyspareunia (pain during intercourse)
- Cervical motion tenderness (in advanced cases)
- Signs of systemic infection (tachycardia, hypotension)
Diagnostic Evaluation and Identification of Anaerobic Peptococcus
Diagnosis of Peptococcus pelvic cellulitis relies on clinical suspicion supported by imaging, laboratory evaluation, and anaerobic microbial identification.
Laboratory Investigations:
- Complete blood count (CBC): Leukocytosis with left shift
- Inflammatory markers: Elevated ESR and CRP
- Blood cultures: May identify anaerobic bacteremia in severe cases
- Vaginal/cervical swabs: Can isolate Peptococcus when cultured anaerobically
Imaging Studies:
- Pelvic Ultrasound
- First-line imaging to detect abscesses or fluid collection
- CT Scan of Pelvis
- Superior in identifying soft tissue swelling, pelvic wall involvement, and deeper abscesses
- MRI of the Pelvis
- Best modality for evaluating spread to surrounding tissues, including fascia and musculature
Differential Diagnosis of Peptococcus Pelvic Infections
Distinguishing Peptococcus pelvic cellulitis from other gynecologic and abdominal conditions is vital for targeted therapy.
| Condition | Differentiating Features |
|---|---|
| Pelvic Inflammatory Disease | Often polymicrobial, includes Neisseria/Gardnerella |
| Pelvic Abscess | Frequently follows untreated cellulitis |
| Appendicitis | Right lower quadrant pain, no vaginal discharge |
| Endometritis | More uterine-focused, postpartum association |
| Diverticulitis | Left-sided, gastrointestinal symptoms predominant |
Microbiology: Peptococcus as a Pathogen in Pelvic Cellulitis
Peptococcus is part of the anaerobic cocci family, typically co-isolated with other anaerobic or facultative organisms such as:
- Bacteroides fragilis
- Prevotella species
- Fusobacterium
- Escherichia coli (in mixed infections)
Successful identification mandates anaerobic culture techniques using special transport and growth conditions. Gram stain typically reveals gram-positive cocci in clusters or short chains.
Treatment Strategies for Peptococcus Pelvic Cellulitis
Effective management involves a multimodal approach combining antibiotic therapy, source control, and supportive care.
Empiric and Targeted Antibiotic Therapy:
Initial therapy should broadly cover anaerobes. Once Peptococcus is confirmed, de-escalation to targeted agents is advised.
First-Line Antibiotics:
- Clindamycin: 600–900 mg IV every 8 hours
- Metronidazole: 500 mg IV/PO every 8 hours
- Ampicillin-Sulbactam: 3 g IV every 6 hours
- Piperacillin-Tazobactam: For polymicrobial infections
Duration of Therapy:
- Minimum 10–14 days IV therapy
- Transition to oral antibiotics for 2–3 weeks depending on clinical response
Surgical and Procedural Interventions:
- Abscess drainage: Transvaginal or transabdominal image-guided aspiration
- Debridement: In cases of necrotizing infection
- IUD removal: If present during infection
- Laparotomy/laparoscopy: For extensive or refractory disease
Complications of Untreated or Inadequately Treated Infections
Failure to manage Peptococcus pelvic cellulitis promptly can lead to significant morbidity.
Possible Complications:
- Pelvic abscess or tubo-ovarian abscess
- Septic shock
- Infertility due to tubal damage
- Chronic pelvic pain
- Fistula formation (rectovaginal or vesicovaginal)
- Disseminated intravascular coagulation (DIC)
Prognosis and Long-Term Outcomes
With early intervention and appropriate therapy, most patients experience full recovery. Delayed treatment increases the risk of chronic complications.
Positive Prognostic Indicators:
- Early diagnosis and antibiotic initiation
- Complete abscess drainage
- Absence of immunosuppression or comorbidities
- Timely surgical intervention when needed
Prevention of Peptococcus Pelvic Cellulitis
Preventive strategies focus on maintaining pelvic health, hygiene, and minimizing procedural risks.
Preventive Measures:
- Sterile technique during gynecological procedures
- Prophylactic antibiotics during surgeries
- Prompt treatment of bacterial vaginosis or STIs
- Safe IUD insertion practices
- Postpartum infection surveillance
Peptococcus pelvic cellulitis is a rare but formidable anaerobic infection necessitating a high index of suspicion. It often arises following pelvic instrumentation, obstetric events, or underlying pelvic disease. Effective management hinges on early detection, targeted antibiotic therapy, and appropriate surgical intervention when required. A structured approach ensures resolution, minimizes recurrence, and preserves reproductive and pelvic organ health.