Esophageal squamous cell carcinoma (ESCC) represents a predominant histological subtype of esophageal cancer, particularly in high-incidence regions across Asia and parts of Africa. It arises from the squamous epithelial lining and is frequently associated with tobacco use, alcohol consumption, dietary factors, and chronic esophageal irritation.
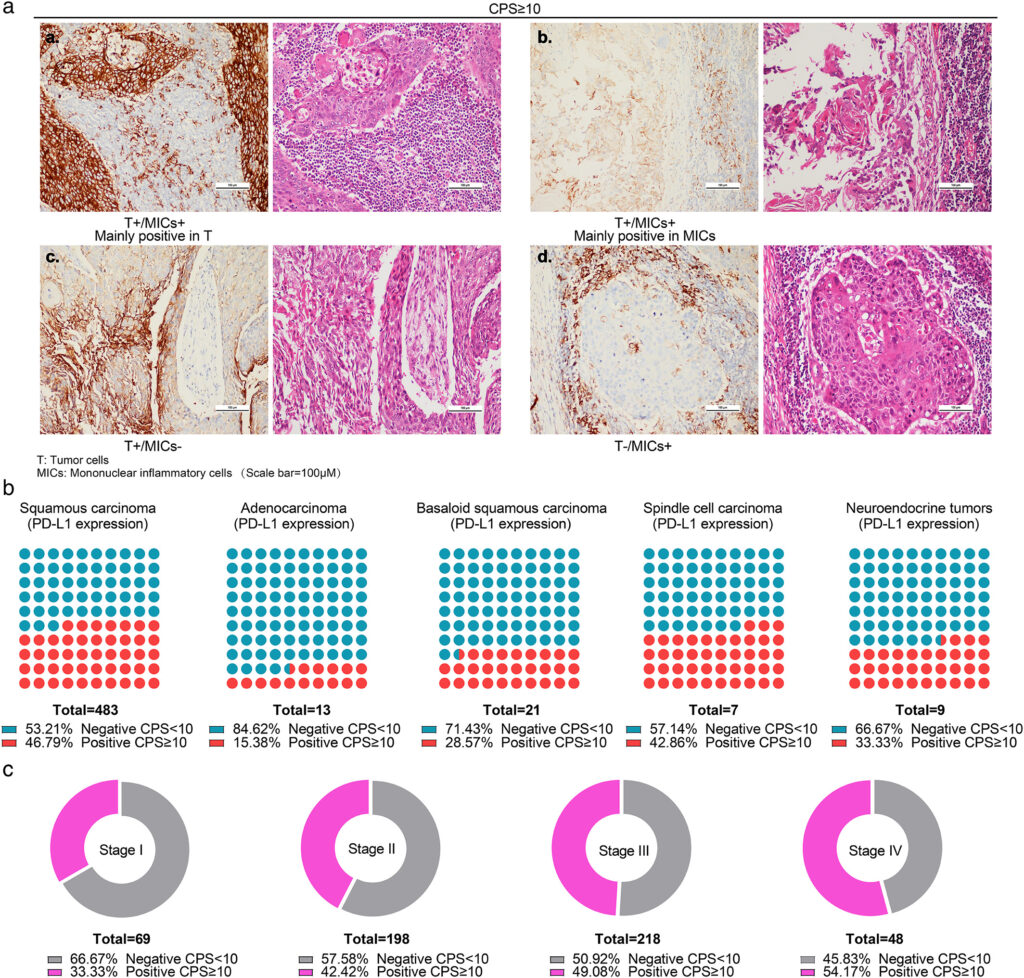
The Role of PD-L1 in ESCC
Programmed death-ligand 1 (PD-L1) is a critical immune checkpoint protein expressed on tumor cells and tumor-infiltrating immune cells. Its interaction with the PD-1 receptor on T-cells leads to immune evasion, allowing tumors to grow unchecked. In ESCC, PD-L1 expression has been identified in a substantial subset of patients, correlating with disease progression and response to immunotherapy.
PD-L1 Testing and Evaluation
Accurate assessment of PD-L1 expression is essential for therapeutic decision-making. Immunohistochemistry (IHC) is the standard technique used, typically employing assays such as:
- 22C3 pharmDx
- SP263 assay
- 28-8 pharmDx
The results are reported using the Combined Positive Score (CPS) or Tumor Proportion Score (TPS), depending on the assay and therapeutic context.
PD-L1 Scoring Systems
- TPS: Percentage of tumor cells with membranous PD-L1 staining
- CPS: [(PD-L1–staining tumor cells + immune cells) / total viable tumor cells] × 100
CPS ≥10 is often used as a cutoff to define PD-L1 positivity in clinical trials.
Clinical Significance of PD-L1 Positivity in ESCC
Prognostic Value
While PD-L1 expression alone does not always predict outcomes, several studies suggest its overexpression may indicate aggressive tumor behavior and potential resistance to conventional therapy.
Predictive Value for Immunotherapy
PD-L1 positivity has emerged as a predictive biomarker for response to immune checkpoint inhibitors. Particularly, it aids in identifying patients who may benefit from PD-1/PD-L1 axis blockade therapies.
Immunotherapy for PD-L1 Positive ESCC
Immune checkpoint inhibitors have revolutionized the treatment landscape for advanced ESCC. Several PD-1 inhibitors are now approved or under evaluation for PD-L1 positive cases.
Nivolumab
Nivolumab, a fully human IgG4 PD-1 inhibitor, has shown remarkable efficacy in several phase III clinical trials.
KEYNOTE & ATTRACTION Trials
- ATTRACTION-3: Demonstrated improved overall survival (OS) in patients with advanced or metastatic ESCC, regardless of PD-L1 status.
- KEYNOTE-590: Pembrolizumab in combination with chemotherapy improved OS and progression-free survival in patients with CPS ≥10.
Pembrolizumab
Another PD-1 inhibitor, Pembrolizumab, has been approved in combination with chemotherapy for first-line treatment in PD-L1 positive cases.
Emerging Therapies and Ongoing Trials
Combination Therapies
- Checkpoint Inhibitors + Chemotherapy: Enhances tumor immunogenicity and improves T-cell infiltration.
- Checkpoint Inhibitors + Anti-angiogenic Agents: Target tumor vasculature and modulate immune suppression.
Novel Biomarkers
Beyond PD-L1, research is exploring other biomarkers like:
- Tumor Mutational Burden (TMB)
- Microsatellite Instability (MSI)
- T-cell inflamed gene expression profiles
These may refine patient selection and optimize outcomes.
Challenges in PD-L1 Guided Therapy
- Heterogeneity in Expression: PD-L1 levels can vary within tumor sites and over time.
- Assay Variability: Different IHC assays and scoring methods complicate standardization.
- Immune-related Adverse Events (irAEs): Require close monitoring and prompt management.
Future Directions
Ongoing clinical trials and translational research are expected to:
- Improve predictive accuracy of PD-L1 and related biomarkers
- Define optimal combination regimens
- Establish long-term survival benefits
- Expand access to immunotherapy in low-resource settings
PD-L1 positive squamous cell carcinoma of the esophagus represents a clinically distinct subset of ESCC with promising responsiveness to immunotherapy. Accurate biomarker testing, thoughtful integration of PD-1/PD-L1 inhibitors, and awareness of emerging evidence are essential for improving outcomes. As our understanding evolves, a more personalized, biomarker-driven approach to ESCC management is becoming the new standard.