Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers and remains a leading cause of cancer-related deaths globally. A subset of these tumors express the immune checkpoint protein PD-L1 (programmed death-ligand 1), which plays a critical role in immune evasion. PD-L1 positive NSCLC has emerged as a clinically relevant biomarker, guiding the use of immune checkpoint inhibitors and defining personalized treatment strategies.
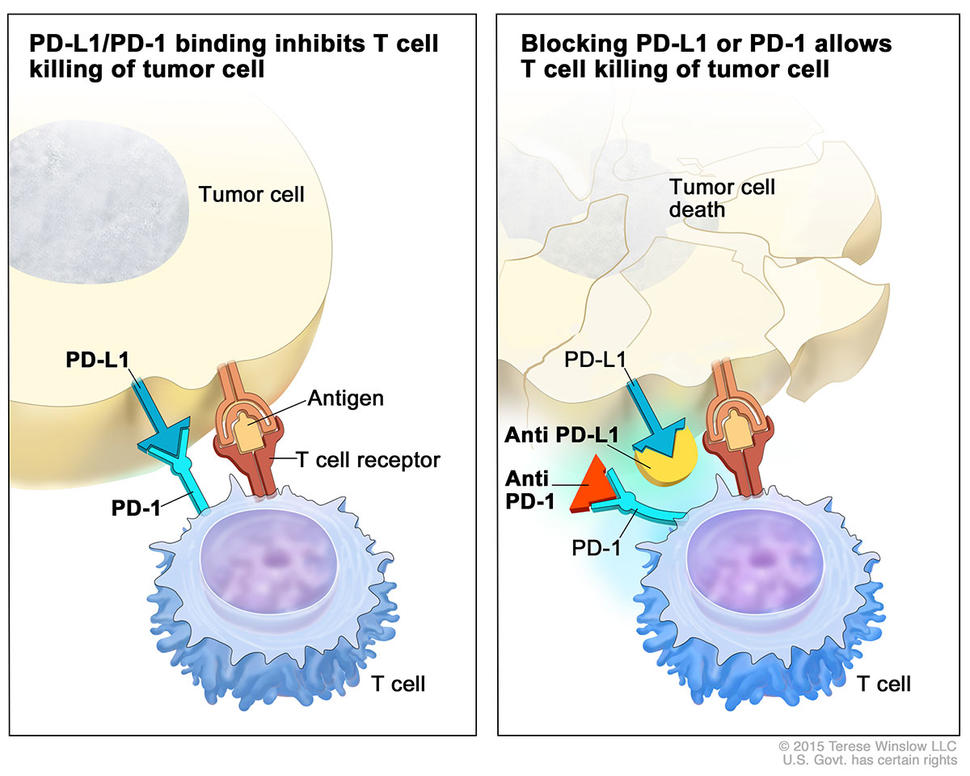
Understanding PD-L1 Expression in NSCLC
The Role of PD-L1 in Tumor Immunology
PD-L1 is a transmembrane protein that binds to the PD-1 receptor on T cells, inhibiting immune responses. In the tumor microenvironment, PD-L1 expression allows cancer cells to evade immune surveillance. In NSCLC, PD-L1 is expressed on both tumor cells and immune-infiltrating cells, creating an immunosuppressive landscape.
Key Points:
- PD-L1 expression is dynamic and influenced by oncogenic signaling, hypoxia, and inflammation.
- Tumors with high PD-L1 levels often show increased immune infiltration, indicating an adaptive immune resistance mechanism.
PD-L1 Testing and Expression Scoring in NSCLC
Diagnostic Tools and Assays
PD-L1 expression in NSCLC is assessed using immunohistochemistry (IHC) assays approved for clinical use, including:
- 22C3 pharmDx (Agilent Technologies)
- SP263 (Ventana)
- 28-8 pharmDx (Dako)
Each assay uses tumor proportion score (TPS) to determine PD-L1 positivity.
| Tumor Proportion Score (TPS) | PD-L1 Status |
|---|---|
| < 1% | Negative |
| ≥ 1% | Positive (low) |
| ≥ 50% | High PD-L1 expression |
Clinical Relevance of TPS Levels
- TPS ≥ 50%: Eligible for monotherapy with anti–PD-1 agents (e.g., pembrolizumab).
- TPS 1–49%: Often treated with immunotherapy plus chemotherapy.
- TPS < 1%: May still benefit from combination regimens or clinical trials.
Approved Immunotherapies for PD-L1 Positive NSCLC
Pembrolizumab (Keytruda)
Pembrolizumab is a PD-1 checkpoint inhibitor approved as:
- First-line monotherapy for advanced NSCLC with TPS ≥ 50%, and no EGFR/ALK alterations.
- Combination therapy with chemotherapy for tumors with TPS ≥ 1%, regardless of driver mutations.
KEYNOTE-024 Trial Highlights:
- Improved overall survival (OS) and progression-free survival (PFS) in patients with high PD-L1 expression.
- Reduced adverse effects compared to chemotherapy alone.
Atezolizumab (Tecentriq)
Approved as:
- First-line monotherapy for patients with high PD-L1 expression (≥50% on TCs or ≥10% on ICs).
- Often combined with bevacizumab and chemotherapy in broader PD-L1 expression profiles.
Cemiplimab (Libtayo)
Monotherapy for patients with:
- Advanced NSCLC and TPS ≥ 50%, based on EMPOWER-Lung 1 trial.
- Demonstrated survival benefits comparable to pembrolizumab.
Biomarker Integration Beyond PD-L1
While PD-L1 remains the cornerstone biomarker, its predictive power is enhanced when considered alongside other biological markers.
Tumor Mutational Burden (TMB)
- High TMB correlates with greater neoantigen load and increased response to checkpoint blockade.
- While not routinely used, TMB is emerging as a complementary biomarker in NSCLC.
Genomic Alterations
- EGFR and ALK mutations are typically less responsive to PD-1/PD-L1 inhibition.
- PD-L1 testing must be integrated with molecular profiling for EGFR, ALK, ROS1, BRAF, KRAS, MET, RET, and HER2.
Frontline Treatment Strategies Based on PD-L1 Levels
| PD-L1 Status (TPS) | Recommended Strategy |
|---|---|
| ≥ 50% | Monotherapy with pembrolizumab or cemiplimab |
| 1–49% | Combination of PD-1 inhibitor with platinum chemo |
| < 1% | Chemo-immunotherapy combinations or clinical trials |
Clinical decision-making also considers:
- Performance status (ECOG PS)
- Comorbidities
- Driver mutations
- Metastatic burden (especially brain/liver involvement)
Resistance to Immunotherapy in PD-L1 Positive NSCLC
Primary and Acquired Resistance
Despite PD-L1 expression, not all patients respond. Resistance mechanisms include:
- Loss of antigen presentation (e.g., B2M mutations)
- T-cell exclusion due to stromal barriers
- Upregulation of alternate checkpoints (e.g., LAG-3, TIM-3)
- Immunosuppressive cell populations (Tregs, MDSCs)
Overcoming Resistance
- Dual checkpoint blockade (e.g., PD-1 + CTLA-4 inhibitors)
- Targeted therapy combinations (e.g., KRAS + ICI)
- Oncolytic viruses, vaccines, or epigenetic modifiers to reinvigorate immune responses
Future Directions in PD-L1 Positive NSCLC Management
Ongoing Clinical Trials
- PD-1 + TIGIT inhibition (e.g., tiragolumab + atezolizumab)
- Triplet regimens combining immunotherapy, anti-angiogenics, and chemotherapy
- Neoantigen-specific T-cell therapies in high TMB or MSI-H tumors
Liquid Biopsy and Dynamic PD-L1 Monitoring
- Circulating tumor DNA (ctDNA) and exosomal PD-L1 are under evaluation for non-invasive monitoring and predicting relapse or response.
PD-L1 positive NSCLC defines a molecularly and immunologically actionable subset of lung cancer, enabling the integration of immunotherapy as a cornerstone of treatment. PD-L1 testing through validated assays guides frontline decisions, while combination strategies expand therapeutic reach. As our understanding of resistance deepens and novel biomarkers emerge, the future of PD-L1 guided therapy promises increasingly precise, durable, and effective outcomes for patients.