Triple-negative breast cancer (TNBC) is a clinically aggressive subtype of breast cancer lacking expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Among TNBCs, those expressing Programmed Death-Ligand 1 (PD-L1) represent a distinct immunogenic subset with unique diagnostic and therapeutic implications.
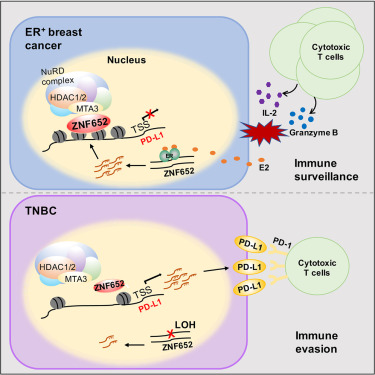
Immunogenicity of PD-L1 Expressing TNBC
Understanding PD-L1 Expression
PD-L1 (CD274) is a transmembrane protein expressed on tumor cells and immune cells within the tumor microenvironment. It binds to the PD-1 receptor on T-cells, inhibiting cytotoxic immune responses and facilitating tumor immune evasion.
In PD-L1 expressing TNBC:
- PD-L1 is often present on tumor-infiltrating immune cells (ICs) and less frequently on tumor cells (TCs)
- Its expression correlates with high tumor-infiltrating lymphocytes (TILs), genomic instability, and immune activation
- It identifies candidates for immune checkpoint blockade therapy
Epidemiology and Molecular Characteristics
Approximately 20% to 40% of TNBCs express PD-L1, particularly in patients with basal-like or immunomodulatory molecular subtypes. Key molecular traits include:
- High tumor mutation burden (TMB)
- Upregulation of interferon gamma (IFN-γ) signaling
- Enrichment in immune cell gene signatures
PD-L1 positivity is more common in younger patients, African American women, and those with high-grade tumors.
Diagnostic Testing for PD-L1 in TNBC
Immunohistochemistry Assays and Scoring
The two primary IHC assays for PD-L1 in TNBC include:
- Ventana SP142: Evaluates PD-L1 expression on immune cells; used in atezolizumab trials
- 22C3 pharmDx: Assesses PD-L1 expression on both tumor and immune cells using the Combined Positive Score (CPS); utilized in pembrolizumab trials
Thresholds for PD-L1 positivity:
| Assay | Biomarker Scoring | Positive Cutoff |
|---|---|---|
| SP142 | IC ≥ 1% | Positive |
| 22C3 pharmDx | CPS ≥ 10 | Positive |
Accurate testing and assay selection are critical for therapeutic decision-making.
Immune Checkpoint Inhibition in PD-L1 Positive TNBC
Atezolizumab (Anti–PD-L1)
Atezolizumab combined with nab-paclitaxel has shown survival benefits in PD-L1 positive metastatic TNBC. Approved based on the IMpassion130 trial, key findings include:
- Progression-free survival (PFS) improvement: 7.5 months vs. 5.0 months
- Overall survival (OS) benefit in PD-L1+ subgroup: 25.0 vs. 15.5 months
Pembrolizumab (Anti–PD-1)
Pembrolizumab, in combination with chemotherapy, is approved for high-risk early-stage and metastatic PD-L1 positive TNBC:
- KEYNOTE-355 (metastatic TNBC): Significant PFS gain for CPS ≥10 patients
- KEYNOTE-522 (early TNBC): Improved pathologic complete response (pCR) when added to neoadjuvant chemotherapy
Therapeutic Implications and Treatment Algorithms
First-Line Therapy for Metastatic PD-L1+ TNBC
- PD-L1+ (SP142+): Atezolizumab + nab-paclitaxel
- PD-L1+ (CPS ≥10): Pembrolizumab + chemotherapy
Neoadjuvant Setting in Early-Stage Disease
- Pembrolizumab + anthracycline-taxane chemotherapy recommended for stage II/III PD-L1+ TNBC
Emerging Options Under Investigation
- Durvalumab (anti–PD-L1)
- Avelumab
- Combination with PARP inhibitors (e.g., olaparib) in BRCA-mutant TNBC
- Vaccine and adoptive T-cell therapies in clinical trials
Predictors of Immunotherapy Response
While PD-L1 status is the dominant biomarker, additional factors influencing immunotherapy outcomes include:
- TIL density: High TILs predict better response
- Tumor Mutational Burden: Elevated TMB enhances neoantigen load
- STING pathway activation: Promotes dendritic cell recruitment
- Microsatellite instability (MSI): Rare in TNBC but predictive when present
Resistance Mechanisms and Limitations
Despite promising efficacy, some PD-L1+ TNBC patients exhibit primary or acquired resistance. Key resistance mechanisms include:
- Loss of antigen presentation
- Immunosuppressive macrophages and regulatory T cells
- Low interferon signaling
- Upregulation of alternative immune checkpoints (e.g., LAG-3, TIM-3)
Combining PD-L1 inhibitors with TGF-β blockers, CD73 antagonists, or oncolytic viruses may overcome resistance.
Long-Term Prognosis and Future Perspectives
PD-L1 expression is now integral to precision oncology in TNBC. Patients with PD-L1 positive tumors receiving checkpoint inhibitors demonstrate:
- Improved pCR rates
- Enhanced survival outcomes
- Durable responses in metastatic disease
Ongoing research aims to refine biomarkers, identify resistance profiles, and optimize combinatorial regimens.
PD-L1 expressing triple-negative breast cancer represents a critical frontier in immunotherapy. Through precise diagnostic stratification and integration of checkpoint inhibitors, we are witnessing a paradigm shift in how TNBC is managed. Continuous clinical trial enrollment and translational research are essential to enhance response rates and ultimately improve survival in this high-risk cohort.