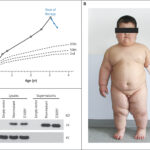
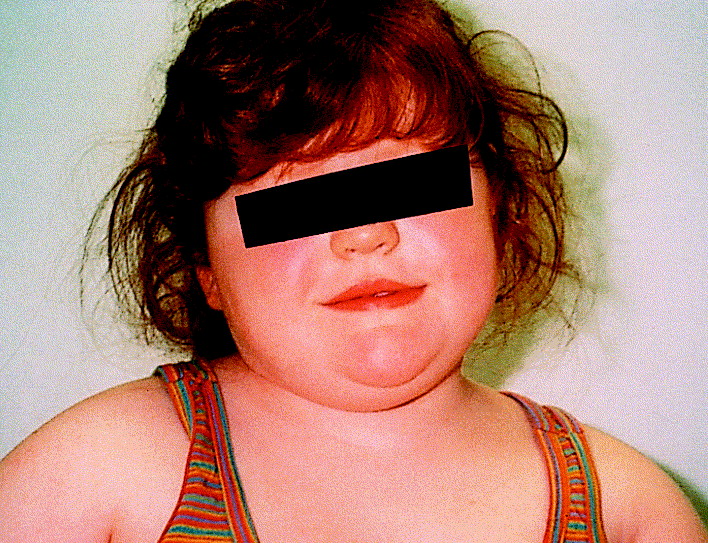
Proopiomelanocortin (POMC) deficiency is a rare form of monogenic obesity characterized by early-onset severe obesity, adrenal insufficiency, and red hair pigmentation in most cases. The disorder stems from biallelic mutations in the POMC gene, disrupting the hypothalamic melanocortin pathway that regulates appetite and energy expenditure. Recognizing and treating this condition early is crucial for improving health outcomes and preventing complications associated with extreme obesity.
Molecular Basis of POMC Deficiency
Role of the POMC Gene in Energy Balance
The POMC gene encodes a precursor polypeptide that undergoes cleavage to produce several peptides, including:
- Adrenocorticotropic hormone (ACTH) – stimulates cortisol production
- Alpha-melanocyte-stimulating hormone (α-MSH) – critical for satiety signaling via the melanocortin 4 receptor (MC4R)
- Beta-endorphin – involved in pain modulation and reward pathways
Mutations in POMC impair the production of these peptides, particularly α-MSH, resulting in disrupted appetite regulation, cortisol deficiency, and distinct pigmentation phenotypes.
Clinical Manifestations of POMC Deficiency
Recognizing the Core Symptoms
Patients with POMC deficiency present with a triad of hallmark features:
- Severe hyperphagia beginning in infancy
- Rapid-onset obesity often noticeable within the first few months of life
- Adrenal insufficiency due to absent or dysfunctional ACTH
- Pale skin and red hair in many but not all patients, due to reduced melanocyte-stimulating hormone activity
Additional manifestations may include hypoglycemia, poor stress response, and delayed developmental milestones secondary to cortisol deficiency.
Diagnostic Evaluation of POMC-Related Obesity
Genetic and Endocrine Workup
Effective diagnosis requires a combination of clinical, biochemical, and genetic assessments:
- Plasma ACTH and cortisol levels – often low or undetectable
- Clinical history of neonatal hypoglycemia and early obesity
- Genetic testing – identification of biallelic pathogenic mutations in POMC confirms the diagnosis
- MC4R pathway functional studies may support therapeutic decisions
Early diagnosis facilitates life-saving steroid replacement and opens the door to precision medicine in appetite regulation.
Pathophysiology: Disrupted Melanocortin Signaling
The primary consequence of POMC deficiency is the absence of α-MSH, which normally binds to the melanocortin 4 receptor (MC4R) in the hypothalamus. This signaling is essential for:
- Suppressing appetite
- Increasing energy expenditure
- Maintaining body weight homeostasis
The lack of α-MSH leads to unchecked appetite (hyperphagia) and excessive fat accumulation, while low ACTH levels cause secondary adrenal insufficiency.
Therapeutic Approaches to POMC Deficiency
Hormonal Replacement for Adrenal Support
Lifelong glucocorticoid replacement therapy (e.g., hydrocortisone) is mandatory to prevent adrenal crises. Regular endocrine monitoring is essential for dose adjustments during stress, illness, or growth spurts.
Setmelanotide: A Breakthrough in Precision Therapy
Setmelanotide, an MC4R agonist, has shown transformative efficacy in treating obesity due to POMC deficiency. By directly stimulating the MC4R, it bypasses the need for α-MSH and restores satiety signaling.
Key benefits of setmelanotide:
- Significant reduction in hunger and caloric intake
- Sustained weight loss without adverse psychiatric effects
- FDA-approved for POMC deficiency-related obesity in patients as young as 6 years old
- Personalized therapy based on confirmed genetic mutations
Long-term data support its role in improving quality of life, body composition, and metabolic health.
Monitoring and Long-Term Management
Multidisciplinary Approach for Holistic Care
Management of POMC deficiency requires coordination across specialties:
- Endocrinology for adrenal function and pubertal development
- Genetics for diagnosis, counseling, and family screening
- Nutrition and psychology to support behavioral adjustments alongside pharmacotherapy
- Pediatrics for developmental tracking and education
Patient and caregiver education on adrenal crisis prevention, stress dosing, and medication adherence is essential.
Prognosis and Quality of Life
With early intervention, patients can lead significantly improved lives. The advent of setmelanotide therapy has changed the trajectory of the condition, offering:
- Improved weight control
- Reduced hunger and food preoccupation
- Normalization of metabolic parameters
- Enhanced psychosocial functioning
Genetic counseling helps families understand reproductive risks and explore prenatal testing options if desired.
Summary of Diagnostic and Treatment Algorithm
- Suspect POMC deficiency in infants with severe early-onset obesity and hypoglycemia
- Conduct cortisol and ACTH testing
- Initiate glucocorticoid therapy for adrenal insufficiency
- Order genetic testing for POMC mutations
- Start setmelanotide therapy upon confirmation
- Implement multidisciplinary care model
Frequently Asked Questions
Q1: What causes obesity in POMC deficiency?
The absence of α-MSH disrupts MC4R signaling in the hypothalamus, leading to severe hyperphagia and early-onset obesity.
Q2: Can red hair be used to diagnose POMC deficiency?
While common, red hair is not a universal sign. Its absence does not exclude the diagnosis.
Q3: Is there a cure for POMC deficiency?
There is no cure, but targeted therapies like setmelanotide offer effective control of hunger and obesity.
Q4: What happens if adrenal insufficiency goes untreated?
Untreated adrenal insufficiency can lead to life-threatening adrenal crises, particularly during illness or stress.
Q5: Who should undergo genetic testing for POMC mutations?
Infants with early obesity, adrenal dysfunction, and a relevant family history should be prioritized for testing.
Obesity due to proopiomelanocortin (POMC) deficiency represents a unique and challenging form of monogenic obesity. It combines endocrine dysfunction, neurological appetite dysregulation, and metabolic complications. Through advances in genetic diagnostics and MC4R-targeted therapy, particularly with setmelanotide, we can provide patients with renewed hope, better health, and a significantly enhanced quality of life. Early recognition and a proactive, personalized approach are critical to changing the outlook for individuals living with this rare but treatable condition.