Neonatal thyrotoxicosis is a rare but potentially life-threatening endocrine disorder in newborns, characterized by excessive thyroid hormone levels within the first month of life. Most commonly, it results from transplacental passage of thyroid-stimulating immunoglobulins (TSIs) from mothers with Graves’ disease. Prompt recognition and treatment are essential to prevent serious complications, including heart failure and developmental delays.
Etiology and Pathophysiology
Primary Cause: Transplacental Transfer of TRAb
The predominant cause of neonatal thyrotoxicosis is the passage of thyroid-stimulating receptor antibodies (TRAb) from a mother with active or previously treated Graves’ disease. These antibodies stimulate the neonatal thyroid gland, leading to excessive production of thyroxine (T4) and triiodothyronine (T3).
Secondary and Rare Causes
- Activating mutations of the thyrotropin receptor (TSHR) gene – autosomal dominant non-autoimmune hyperthyroidism
- Maternal chorionic gonadotropin-secreting tumors
- Iatrogenic causes due to maternal treatment with thyroid hormones
Risk Factors
- Maternal history of Graves’ disease (even if treated surgically or with radioiodine)
- Elevated maternal TRAb levels during the third trimester
- Sibling with prior neonatal thyrotoxicosis
- Preterm birth or low birth weight may exacerbate severity
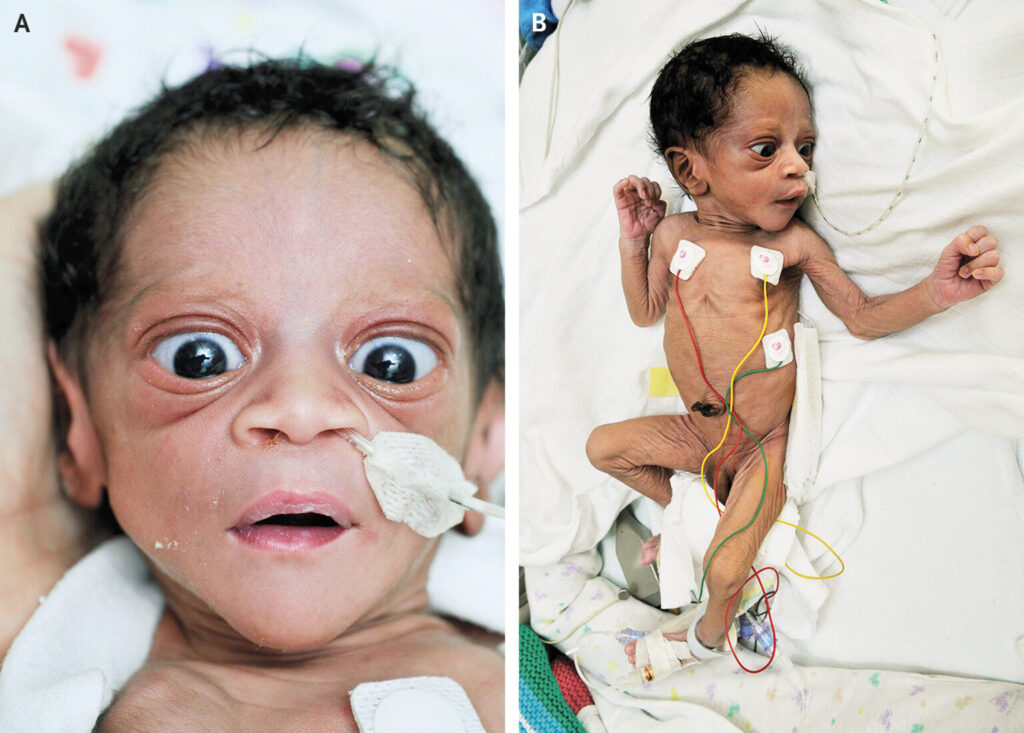
Clinical Manifestations
Symptoms typically appear within the first few days of life but may be delayed up to two weeks postpartum due to maternal anti-thyroid drug (ATD) exposure during pregnancy.
Common Signs and Symptoms
- Tachycardia (heart rate >180 bpm)
- Irritability and poor feeding
- Failure to thrive
- Goiter – visible or palpable thyroid enlargement
- Exophthalmos (rare in neonates)
- Warm, moist skin
- Hepatosplenomegaly
- Hypertension
- Advanced bone age
Severe Complications
- High-output congestive heart failure
- Arrhythmias
- Craniosynostosis
- Accelerated growth followed by growth failure
- Neurodevelopmental impairment if untreated
Diagnostic Approach
Timely diagnosis hinges on clinical suspicion, maternal history, and confirmatory laboratory tests.
Key Laboratory Tests
| Test | Interpretation |
|---|---|
| Serum TSH | Suppressed or undetectable |
| Free T4 and T3 | Elevated |
| Thyroid receptor antibodies (TRAb) | Positive in both mother and neonate |
| Thyroid ultrasound | May show increased vascularity and enlarged gland |
Additional Evaluations
- ECG/Echocardiogram: Assess for arrhythmias or cardiomegaly
- Liver function tests: May be abnormal due to congestive hepatopathy
- Bone age assessment: Advanced skeletal maturation may be evident
Management and Treatment Strategies
Management involves controlling thyroid hormone levels, monitoring systemic effects, and treating the underlying immunological cause.
First-Line Therapies
- Methimazole (preferred antithyroid drug): 0.5–1.5 mg/kg/day divided every 8 hours
- Propranolol: 2 mg/kg/day divided to control tachycardia and adrenergic symptoms
- Iodine solution (Lugol’s iodine): Temporarily inhibits thyroid hormone release (used selectively)
Corticosteroids
- Hydrocortisone: Reduces peripheral T4 to T3 conversion and suppresses TRAb production
Supportive Care
- Fluid and electrolyte balance
- Nutritional support
- Oxygen and cardiac monitoring in severe cases
Treatment Duration
Typically, symptoms resolve as maternal TRAb levels wane—within 2 to 3 months. Antithyroid therapy is gradually tapered based on thyroid function tests.
Monitoring and Follow-Up
Frequent thyroid function monitoring is essential:
| Timeframe | Tests |
|---|---|
| Initial diagnosis | Daily TSH, Free T4, Total T3 |
| During treatment | Every 2–3 days initially, then weekly |
| Post-resolution | Monthly for 6–12 months to detect hypothyroidism or relapse |
Neurodevelopmental evaluations should be integrated into pediatric follow-up to assess for any long-term deficits.
Prognosis and Long-term Outcomes
Short-Term
With timely intervention, the prognosis is favorable. Untreated or delayed cases are at risk of:
- Cardiac failure
- Seizures
- Death
Long-Term
- Most infants return to a euthyroid state once maternal antibodies are cleared
- Rarely, persistent hyperthyroidism may indicate a non-autoimmune etiology
- Neurodevelopment is typically normal with prompt management, though subtle cognitive issues have been reported
Prevention and Antenatal Considerations
Maternal Monitoring
- TRAb Testing at 20–24 and 30–34 weeks’ gestation
- Close monitoring of fetal heart rate, growth, and goiter via fetal ultrasound
Fetal Therapy
In utero therapy with maternal methimazole may be considered in severe fetal thyrotoxicosis (e.g., fetal tachycardia, hydrops).
Differentiating from Other Conditions
| Condition | Features | Distinguishing Factor |
|---|---|---|
| Neonatal Sepsis | Fever, tachycardia, lethargy | Inflammatory markers elevated, infection confirmed |
| Congenital Adrenal Hyperplasia | Vomiting, dehydration | Hyponatremia, hyperkalemia |
| Congenital Hyperinsulinism | Hypoglycemia, jitteriness | Low blood glucose, insulin elevated |
Frequently Asked Questions
What causes neonatal thyrotoxicosis?
It is most often due to maternal Graves’ disease, where thyroid-stimulating antibodies cross the placenta.
Is neonatal thyrotoxicosis permanent?
No. It is usually transient and resolves as maternal antibodies diminish in the infant’s bloodstream.
Can it occur if the mother had treatment for Graves’ disease?
Yes. Even after thyroidectomy or radioactive iodine treatment, TRAb can persist and affect the fetus.
When do symptoms appear?
Symptoms may appear within the first few days but can be delayed up to two weeks postpartum.
Can neonatal thyrotoxicosis be prevented?
While not entirely preventable, careful monitoring and management of maternal Graves’ disease significantly reduce risk.
Neonatal thyrotoxicosis, though rare, demands early detection and prompt intervention due to its potential severity. Maternal history and TRAb screening play critical roles in prenatal risk assessment. With coordinated care between obstetrics, neonatology, and pediatric endocrinology, affected neonates can achieve excellent outcomes with minimal long-term effects.

