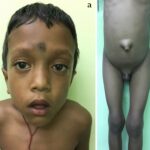
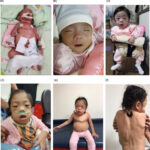
Mucopolysaccharidosis type IV-A (MPS IV-A), also known as Morquio A syndrome, is a rare lysosomal storage disorder caused by a deficiency of the enzyme N-acetylgalactosamine-6-sulfatase (GALNS). This enzyme is essential for breaking down glycosaminoglycans (GAGs), specifically keratan sulfate and chondroitin-6-sulfate. The accumulation of these substances leads to progressive skeletal abnormalities and systemic complications.
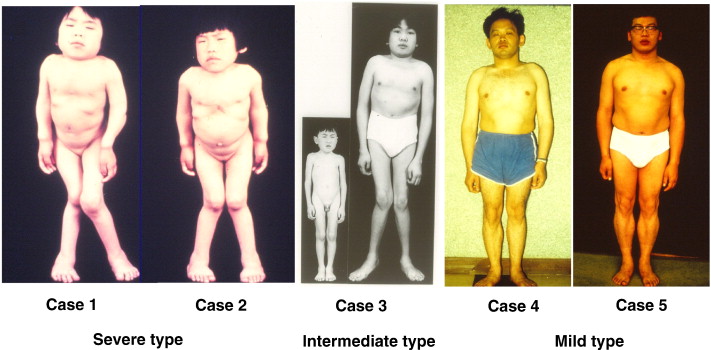
What is Mucopolysaccharidosis Type IV-A?
MPS IV-A belongs to a group of mucopolysaccharidoses, characterized by impaired degradation of glycosaminoglycans. It follows an autosomal recessive inheritance pattern, meaning that an affected individual inherits two defective copies of the GALNS gene, one from each parent.
Types of Morquio Syndrome
MPS IV consists of two subtypes:
| Type | Deficient Enzyme | Severity | Main Symptoms |
|---|---|---|---|
| MPS IV-A (Morquio A) | GALNS deficiency | Severe | Skeletal dysplasia, joint instability, short stature |
| MPS IV-B (Morquio B) | β-galactosidase deficiency | Milder | Similar skeletal symptoms but less severe |
Causes and Genetic Basis of MPS IV-A
MPS IV-A is caused by mutations in the GALNS gene, located on chromosome 16q24.3. These mutations impair the function of N-acetylgalactosamine-6-sulfatase, leading to the accumulation of keratan sulfate in bones, cartilage, and connective tissue.
Symptoms of MPS IV-A
MPS IV-A symptoms typically become apparent between ages 1 and 3 years, with progressive worsening over time. Unlike other mucopolysaccharidoses, MPS IV-A does not significantly affect intelligence.
Early Signs and Symptoms
- Delayed walking or abnormal gait
- Short stature and slow growth
- Joint laxity (hypermobile joints)
- Flattened vertebrae (platyspondyly)
Skeletal and Musculoskeletal Symptoms
- Severe skeletal dysplasia affecting the spine, chest, and limbs
- Kyphoscoliosis (curvature of the spine)
- Genu valgum (knock-knees)
- Hip dysplasia and early-onset arthritis
Respiratory and Cardiovascular Symptoms
- Airway obstruction and sleep apnea
- Restricted lung function due to skeletal abnormalities
- Heart valve disease (mitral/aortic regurgitation)
Ophthalmological and Hearing Issues
- Corneal clouding
- Hearing loss (conductive or sensorineural)
Neurological and Spinal Complications
- Spinal cord compression (cervical myelopathy)
- Increased risk of paralysis if untreated
Diagnosis of MPS IV-A
Clinical Examination and Medical History
Physicians assess:
- Growth patterns and skeletal abnormalities
- Joint hypermobility and ligamentous laxity
Laboratory and Genetic Tests
- Enzyme Assay: Measures GALNS enzyme activity in blood or fibroblasts.
- Urinary Glycosaminoglycan (GAG) Analysis: Detects elevated keratan sulfate.
- Genetic Testing: Identifies GALNS mutations.
Imaging and Other Diagnostic Tests
- X-rays: Reveal skeletal dysplasia and vertebral abnormalities.
- MRI of the Spine: Evaluates cervical cord compression.
- Echocardiogram: Assesses heart valve function.
Treatment Options for MPS IV-A
There is no cure for MPS IV-A, but early intervention can significantly improve quality of life.
1. Enzyme Replacement Therapy (ERT)
Elosulfase alfa (Vimizim) is an FDA-approved enzyme replacement therapy for MPS IV-A.
- Administered via intravenous infusion weekly.
- Improves endurance and reduces keratan sulfate buildup.
- Does not cross the blood-brain barrier, limiting its effects on neurological complications.
2. Supportive and Symptomatic Treatments
- Orthopedic Surgeries: Correct spinal deformities, hip dysplasia, and knee abnormalities.
- Physical Therapy: Helps maintain mobility and reduce joint stiffness.
- Respiratory Support: CPAP or tracheostomy for severe airway obstruction.
- Hearing Aids: Manage progressive hearing loss.
3. Surgical Interventions
- Spinal Decompression Surgery: Prevents paralysis due to cervical myelopathy.
- Corneal Transplant: Treats severe corneal clouding.
- Cardiac Surgery: Valve replacement if heart disease progresses.
Prognosis and Life Expectancy
Life expectancy varies depending on the severity of skeletal and respiratory complications:
- Severe MPS IV-A: Life expectancy is typically 20-30 years, primarily due to respiratory failure.
- Milder Cases: Some individuals survive into their 40s or 50s with medical management.
Research and Emerging Therapies
Current research aims to improve treatment options:
- Gene Therapy: Investigating ways to introduce a functional GALNS gene into cells.
- Substrate Reduction Therapy (SRT): Exploring drugs that limit keratan sulfate production.
- Chaperone Therapy: Developing small molecules that stabilize GALNS enzyme activity.
Mucopolysaccharidosis type IV-A (Morquio A syndrome) is a progressive skeletal disorder caused by GALNS enzyme deficiency. While enzyme replacement therapy and supportive treatments can manage symptoms, ongoing research offers hope for more effective therapies. Early diagnosis and intervention are critical to improving long-term outcomes.