Molybdenum cofactor deficiency type A (MoCD-A) is a rare autosomal recessive metabolic disorder that disrupts the function of molybdenum-dependent enzymes, particularly sulfite oxidase. This deficiency leads to severe neurological symptoms, metabolic encephalopathy, and early mortality if left untreated. Early diagnosis and treatment are critical for improving patient outcomes.
Causes and Genetic Basis
MoCD-A is caused by mutations in the MOCS1 gene, which encodes enzymes involved in molybdenum cofactor (MoCo) biosynthesis. MoCo is essential for the activation of several enzymes, including sulfite oxidase, xanthine dehydrogenase, and aldehyde oxidase. Without MoCo, the body accumulates toxic sulfite, leading to severe neurodegeneration.
Symptoms of MoCD-A
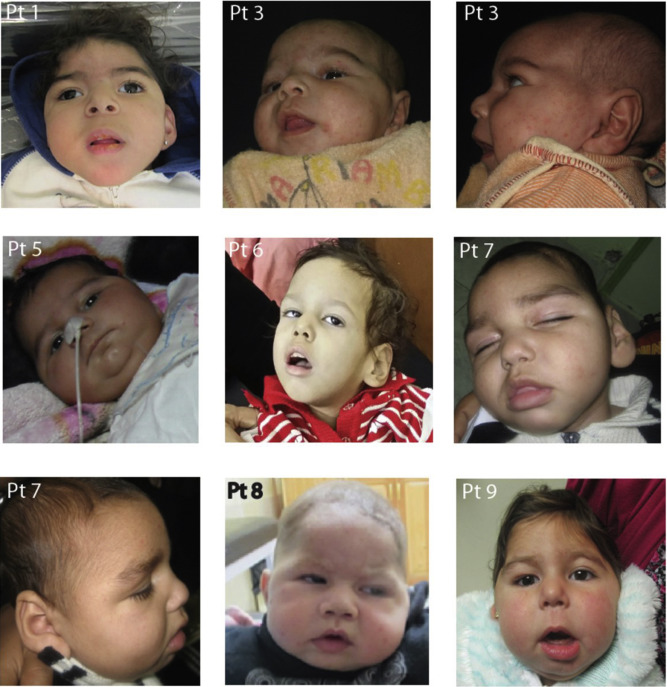
Symptoms of MoCD-A typically appear in the neonatal period and rapidly progress. Common clinical manifestations include:
- Severe encephalopathy
- Intractable seizures
- Hypotonia (low muscle tone)
- Feeding difficulties
- Progressive brain atrophy
- Developmental delays
Diagnosis
Laboratory and Genetic Testing
Diagnosing MoCD-A involves multiple approaches:
- Biochemical Markers: High levels of sulfite and S-sulfocysteine in urine indicate sulfite oxidase deficiency.
- Neuroimaging: MRI scans reveal brain atrophy and white matter abnormalities.
- Genetic Testing: Identifying mutations in the MOCS1 gene confirms the diagnosis.
Treatment Options
Enzyme Replacement Therapy
The primary treatment for MoCD-A is cyclic pyranopterin monophosphate (cPMP) therapy, which bypasses the metabolic block and restores MoCo production. Early administration of cPMP significantly improves survival and neurological outcomes.
Supportive Care
- Seizure Management: Anti-epileptic medications may be used to control seizures.
- Nutritional Support: Tube feeding may be required for infants with feeding difficulties.
- Physical Therapy: Helps manage muscle tone abnormalities and improve mobility.
Prognosis
Without treatment, MoCD-A leads to rapid neurological deterioration and early death. However, with early diagnosis and cPMP therapy, affected individuals may experience significant improvements in neurological function and quality of life.
Molybdenum cofactor deficiency type A is a severe metabolic disorder that requires early diagnosis and targeted treatment. Advances in enzyme replacement therapy, particularly with cPMP, have provided hope for affected individuals. Continued research and newborn screening programs are essential for improving patient outcomes.