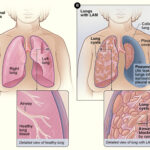
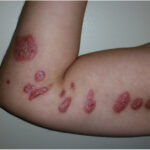
Lymphoblastic lymphoma (LBL) is a rare and aggressive subtype of non-Hodgkin’s lymphoma (NHL), primarily affecting children, adolescents, and young adults. It originates from immature lymphocytes (lymphoblasts) and is closely related to acute lymphoblastic leukemia (ALL). LBL is classified into:
- T-cell lymphoblastic lymphoma (T-LBL) – More common, aggressive, and often involves the mediastinum.
- B-cell lymphoblastic lymphoma (B-LBL) – Less frequent, typically presenting in lymph nodes or bone marrow.
Early diagnosis and intensive chemotherapy are crucial for improving survival rates.
Pathophysiology of Lymphoblastic Lymphoma
LBL arises due to genetic mutations that cause uncontrolled proliferation of immature lymphoblasts. These malignant cells infiltrate lymphoid tissues, bone marrow, and other organs, leading to systemic symptoms.
Causes and Risk Factors
While the exact cause remains unknown, several factors contribute to LBL development:
- Genetic Mutations – Alterations in NOTCH1, TAL1, and TCR genes are linked to T-LBL.
- Chromosomal Abnormalities – Translocations such as t(9;22) (Philadelphia chromosome) are associated with poor prognosis.
- Radiation Exposure – High doses of radiation increase the risk of lymphoid malignancies.
- Immune System Deficiencies – Patients with HIV/AIDS or undergoing immunosuppressive therapy are more vulnerable.
- Environmental Factors – Exposure to benzene, pesticides, or chemotherapy drugs may contribute to lymphoblastic lymphoma.
Signs and Symptoms of Lymphoblastic Lymphoma
LBL symptoms vary based on disease extent but often include:
- Enlarged Lymph Nodes (Lymphadenopathy) – Painless swelling, often in the neck, chest, or groin.
- Mediastinal Mass (T-LBL) – Can cause shortness of breath, cough, and chest pain.
- Bone Marrow Involvement – Leads to anemia, thrombocytopenia, and recurrent infections.
- B Symptoms (Systemic Symptoms) –
✔ Unexplained fever
✔ Night sweats
✔ Unintentional weight loss - Central Nervous System (CNS) Involvement – Headaches, seizures, or neurological deficits occur in advanced stages.
Diagnosis of Lymphoblastic Lymphoma
1. Blood Tests and Bone Marrow Biopsy
✔ Complete Blood Count (CBC) – Checks for anemia, leukocytosis, or thrombocytopenia.
✔ Lactate Dehydrogenase (LDH) Levels – Elevated in aggressive lymphomas.
✔ Bone Marrow Aspiration/Biopsy – Confirms lymphoma infiltration.
2. Immunophenotyping (Flow Cytometry)
Identifies the lymphoma subtype by detecting T-cell (CD3, CD7) or B-cell (CD19, CD22) markers.
3. Imaging Studies
✔ CT/PET Scans – Evaluate lymph node and organ involvement.
✔ MRI (For CNS Involvement) – Detects brain or spinal cord infiltration.
4. Lumbar Puncture
Cerebrospinal fluid (CSF) analysis determines CNS involvement, common in advanced cases.
5. Genetic and Molecular Testing
Detects chromosomal abnormalities like t(9;22) (Philadelphia chromosome), which affect treatment strategy.
Staging of Lymphoblastic Lymphoma
LBL is classified using the Lugano Staging System:
1️⃣ Stage I – Single lymph node region involved.
2️⃣ Stage II – Multiple lymph nodes on the same side of the diaphragm.
3️⃣ Stage III – Lymph node involvement on both sides of the diaphragm.
4️⃣ Stage IV – Dissemination to bone marrow, CNS, or other organs.
Treatment Options for Lymphoblastic Lymphoma
1. Intensive Chemotherapy (Multi-Agent Regimens)
The standard treatment follows ALL-like chemotherapy protocols, consisting of:
✔ Induction Therapy – High-dose corticosteroids, vincristine, anthracyclines, and asparaginase eliminate most cancer cells.
✔ Consolidation Therapy – Cyclophosphamide and cytarabine prevent relapse.
✔ Maintenance Therapy – Lower-dose chemotherapy for prolonged disease control.
2. CNS Prophylaxis
Since LBL frequently spreads to the brain and spinal cord, intrathecal chemotherapy (methotrexate, cytarabine) is essential.
3. Targeted Therapy
✔ Tyrosine Kinase Inhibitors (TKIs) – Imatinib or dasatinib for Philadelphia chromosome-positive LBL.
✔ Monoclonal Antibodies – Blinatumomab (CD19-targeted) improves outcomes in B-LBL.
4. Radiation Therapy
Used for:
✔ Large mediastinal masses causing airway compression
✔ CNS involvement
✔ Palliative care in advanced cases
5. Stem Cell Transplantation
For high-risk or relapsed LBL, autologous or allogeneic hematopoietic stem cell transplantation (HSCT) offers a potential cure.
Prognosis and Survival Rates
LBL prognosis depends on age, disease stage, and response to therapy. Survival rates include:
- Children & Adolescents – 80–90% 5-year survival with chemotherapy.
- Adults – 40–60% 5-year survival, worse in older patients or relapsed cases.
- Relapsed/Refractory LBL – 20–30% survival, requiring aggressive salvage therapy.
Future Research and Emerging Treatments
1. CAR-T Cell Therapy
Chimeric Antigen Receptor (CAR) T-cell therapy is being tested for refractory B-LBL, targeting CD19/CD22 antigens.
2. Bispecific Antibodies
New agents like tebentafusp (for T-LBL) enhance immune response against lymphoma cells.
3. Personalized Medicine
Gene-editing techniques (e.g., CRISPR for TSC1 mutations) may revolutionize targeted therapy.
Frequently Asked Questions:
1. Is Lymphoblastic Lymphoma the Same as Leukemia?
LBL is closely related to acute lymphoblastic leukemia (ALL), but LBL primarily affects lymph nodes, while ALL primarily involves the bone marrow.
2. What Is the Best Treatment for LBL?
Multi-agent chemotherapy is the standard treatment, often combined with CNS prophylaxis and targeted therapy for high-risk cases.
3. Can Lymphoblastic Lymphoma Be Cured?
Yes, 80-90% of children achieve remission, but adults face higher relapse risks. Stem cell transplantation improves survival in relapsed cases.
4. How Fast Does LBL Progress?
LBL is highly aggressive, requiring urgent treatment to prevent rapid disease spread.
5. What Are the Signs of Relapse?
✔ New lymph node swelling
✔ Recurrent fever, night sweats
✔ Bone pain or CNS symptoms