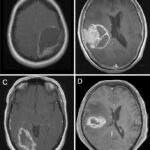
Low-grade gliomas (LGGs) are slow-growing brain tumors commonly found in children and young adults. A subset of these tumors is associated with BRAF fusion or mutation, which significantly influences their behavior and response to treatment. Understanding the molecular characteristics of these tumors helps in formulating targeted therapies, improving patient outcomes.
What is a Low-Grade Glioma?
Low-grade gliomas are classified as grade I or II gliomas based on the World Health Organization (WHO) grading system. They originate from glial cells and typically have a better prognosis compared to high-grade gliomas.
Common Types of LGG
- Pilocytic Astrocytoma (PA) – Most frequently seen in children, often associated with BRAF fusion.
- Diffuse Astrocytoma (DA) – More common in adults, may harbor BRAF mutations.
- Gangliogliomas – A mixed neuronal-glial tumor, sometimes carrying BRAF V600E mutations.
- Pleomorphic Xanthoastrocytoma (PXA) – Often linked to BRAF mutations, but can progress to a higher grade.
Understanding BRAF Fusion and Mutation in LGG
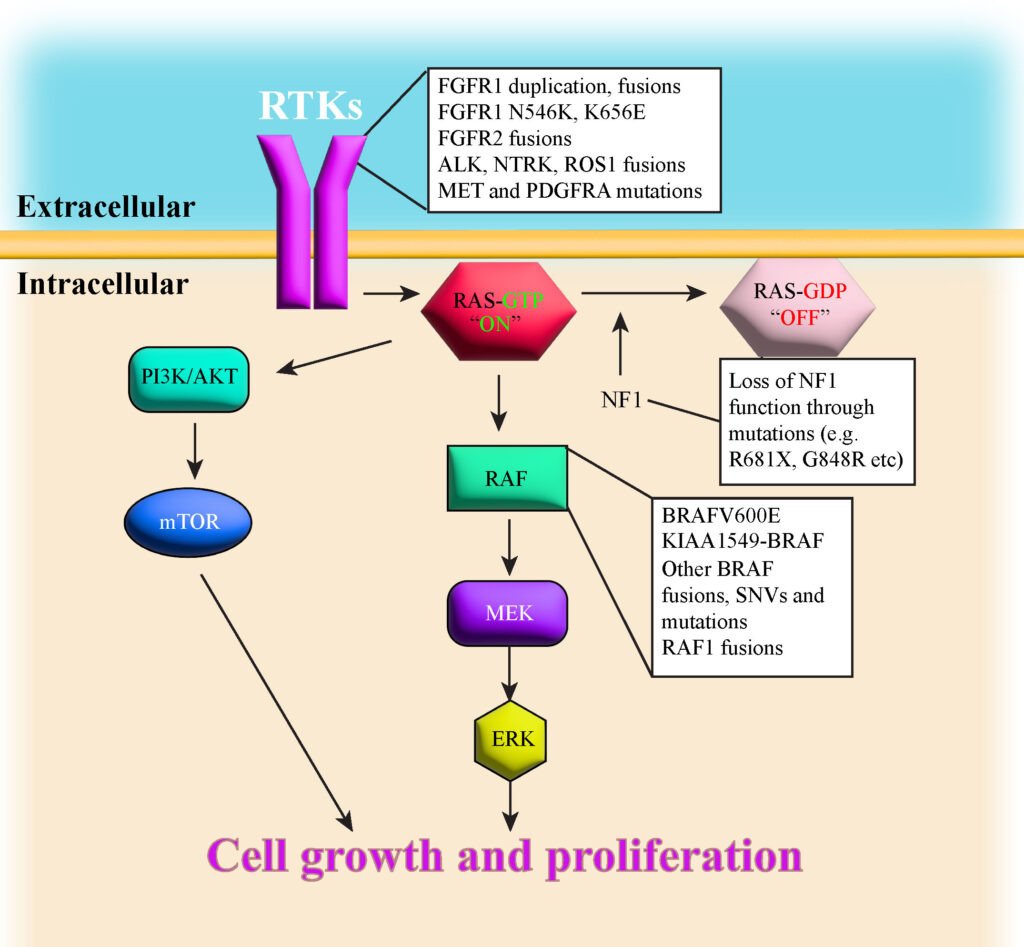
BRAF Fusion
BRAF fusion results from a genetic rearrangement where the BRAF gene fuses with another gene, typically KIAA1549. This leads to uncontrolled cell growth, contributing to tumor development. It is primarily seen in pilocytic astrocytomas and is associated with a better response to targeted therapies.
BRAF V600E Mutation
BRAF mutations, particularly V600E, involve a substitution of valine with glutamic acid at codon 600, leading to continuous activation of the MAPK/ERK pathway. This mutation is observed in gangliogliomas, PXAs, and diffuse astrocytomas, and it can be more aggressive than BRAF fusions.
Symptoms of Low-Grade Glioma with BRAF Alterations
The symptoms of LGGs depend on their location in the brain. Some common manifestations include:
- Headaches – Persistent and progressive, often worse in the morning.
- Seizures – Common in gangliogliomas and PXAs.
- Cognitive and Behavioral Changes – Memory loss, personality changes, or confusion.
- Vision Problems – Blurred or double vision if the tumor affects the optic pathway.
- Weakness or Numbness – Indicating motor cortex involvement.
- Increased Intracranial Pressure (ICP) – Leading to nausea, vomiting, and papilledema.
Diagnosis of BRAF-Associated LGG
Accurate diagnosis is crucial for determining the best treatment approach. Diagnostic steps include:
- Magnetic Resonance Imaging (MRI) – Provides detailed images to locate and characterize the tumor.
- Biopsy and Histopathology – Confirms tumor type and grade.
- Molecular Testing – Identifies BRAF fusion or mutations to guide therapy.
- Positron Emission Tomography (PET) Scan – Helps differentiate low-grade from high-grade tumors.
Treatment Options for Low-Grade Gliomas with BRAF Alterations
Treatment decisions depend on factors such as tumor location, patient age, and genetic profile.
1. Surgical Resection
- Complete Resection – Achieves the best prognosis if the tumor is in an accessible location.
- Subtotal Resection – May be performed if the tumor is near critical brain structures.
2. Targeted Therapy
- BRAF Inhibitors (Dabrafenib, Vemurafenib) – Effective in BRAF V600E-mutated gliomas.
- MEK Inhibitors (Trametinib, Selumetinib) – Used for tumors resistant to BRAF inhibitors or with BRAF fusion.
3. Chemotherapy
- Used when surgery is not possible or when tumors recur.
- Common regimens include carboplatin and vincristine.
4. Radiation Therapy
- Reserved for older patients or when the tumor is unresectable.
- Proton beam therapy is a newer approach with reduced side effects.
5. Observation
- For small, asymptomatic tumors, regular MRI scans are recommended to monitor growth.
Prognosis and Long-Term Outlook
The prognosis for BRAF-associated LGGs is generally favorable, especially with early intervention. Key factors influencing outcomes include:
- Complete surgical removal improves survival.
- BRAF fusion tumors respond well to targeted therapy.
- BRAF V600E-mutated gliomas may have a slightly higher risk of progression.
- Regular follow-ups are essential to detect recurrence early.
Future Research and Emerging Therapies
Ongoing clinical trials are investigating novel therapies such as:
- Next-generation BRAF inhibitors with fewer side effects.
- Combination therapy using BRAF and MEK inhibitors.
- Immunotherapy approaches for LGGs.
Low-grade gliomas with BRAF fusion or mutation represent a distinct subset of brain tumors with specific molecular characteristics. Advances in targeted therapy and precision medicine have significantly improved patient outcomes. Early diagnosis and personalized treatment remain key to managing this condition effectively.