Lamivudine, a nucleoside analog, has been a cornerstone in the treatment of chronic hepatitis B virus (HBV) infection. However, prolonged use has led to the emergence of lamivudine-resistant HBV strains, characterized by mutations in the viral polymerase gene, notably at positions rtL180M and rtM204V/I. These mutations diminish the drug’s efficacy, resulting in viral rebound and potential disease progression.
Identifying Treatment Failure in Lamivudine Therapy
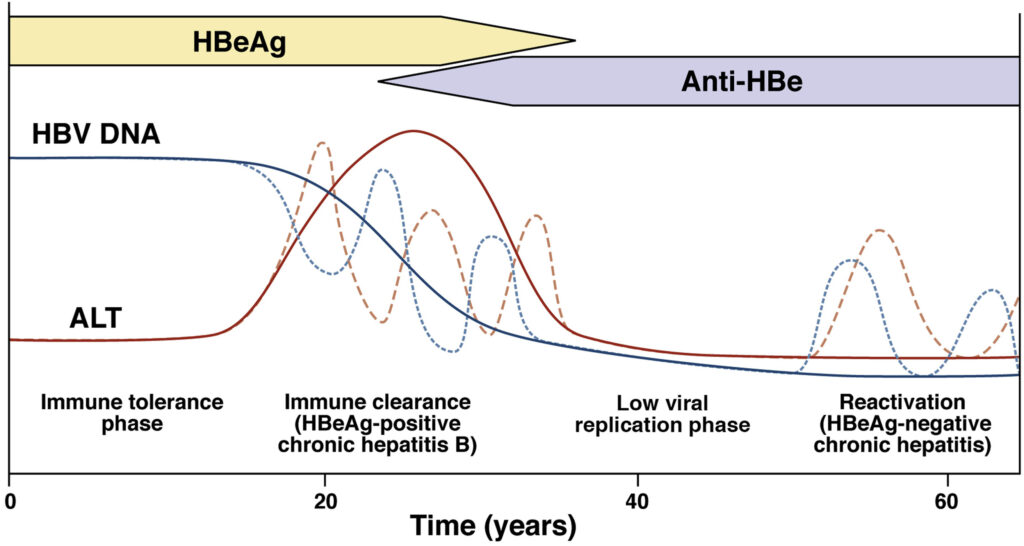
Treatment failure manifests as a virological breakthrough, indicated by a rebound increase in HBV DNA levels after an initial decline. This can be followed by biochemical breakthrough, evidenced by elevated alanine aminotransferase (ALT) levels. Severe cases may present as acute hepatic flares, decompensation in cirrhotic patients, or even fulminant hepatic failure. Regular monitoring of HBV DNA and ALT levels is crucial to detect and address treatment failure promptly.
Alternative Antiviral Agents for Lamivudine-Resistant HBV
In cases of lamivudine resistance, switching to more potent antiviral agents with a higher barrier to resistance is recommended. Entecavir and tenofovir are two such agents that have demonstrated efficacy in this context.
Entecavir: Efficacy and Considerations
Entecavir, a guanosine nucleoside analog, has shown potent antiviral activity against lamivudine-resistant HBV. A phase III, double-blind trial demonstrated that patients switching to entecavir experienced superior histologic improvement and viral load reduction compared to those continuing lamivudine. Specifically, 55% of entecavir-treated patients achieved histologic improvement, and there was a mean HBV DNA reduction of 5.11 log₁₀ copies/mL. However, entecavir’s efficacy may be compromised in the presence of lamivudine-resistant mutations, and its use in such cases requires careful consideration.
Tenofovir: The Preferred Second-Line Therapy
Tenofovir, a nucleotide analog, is recommended as the preferred second-line therapy for patients with confirmed or suspected lamivudine resistance. Despite limited direct evidence from randomized controlled trials, tenofovir has shown the highest probability of achieving undetectable HBV DNA levels in this patient population. Its use avoids the selection of further compensatory mutations and development of multidrug-resistant HBV strains. Additionally, tenofovir’s availability and affordability make it a viable option in various healthcare settings.
Combination Therapy: Evaluating the Benefits
Combination therapy, such as adding adefovir to lamivudine, has been explored as a strategy to manage lamivudine resistance. While some studies suggest that combination therapy may improve viral suppression, the overall benefit remains uncertain. The use of potent monotherapy with agents like tenofovir is generally preferred due to simplicity and efficacy.
Preventing Lamivudine Resistance: Strategies and Recommendations
Preventing the development of lamivudine resistance is paramount. Strategies include:
- Thorough Patient Assessment: Evaluate risk factors such as age, baseline HBV DNA levels, and potential for long-term adherence before initiating therapy.
- Patient Education: Inform patients about the importance of adherence to therapy and the risks associated with non-compliance.
- Regular Monitoring: Conduct periodic assessments of HBV DNA and ALT levels to detect early signs of resistance or treatment failure.
- Appropriate First-Line Therapy Selection: Utilize antiviral agents with a high barrier to resistance, such as entecavir or tenofovir, as initial treatment options to reduce the likelihood of resistance development.
Managing lamivudine-refractory chronic hepatitis B requires a strategic approach, emphasizing the use of potent antiviral agents with high barriers to resistance. Regular monitoring and patient education are critical components in optimizing treatment outcomes and preventing disease progression.