Influenza A (H1N1), commonly known as swine flu, is a highly contagious respiratory virus that caused a global pandemic in 2009. Vaccination is the most effective method to prevent infection and mitigate severe symptoms associated with this subtype of influenza.
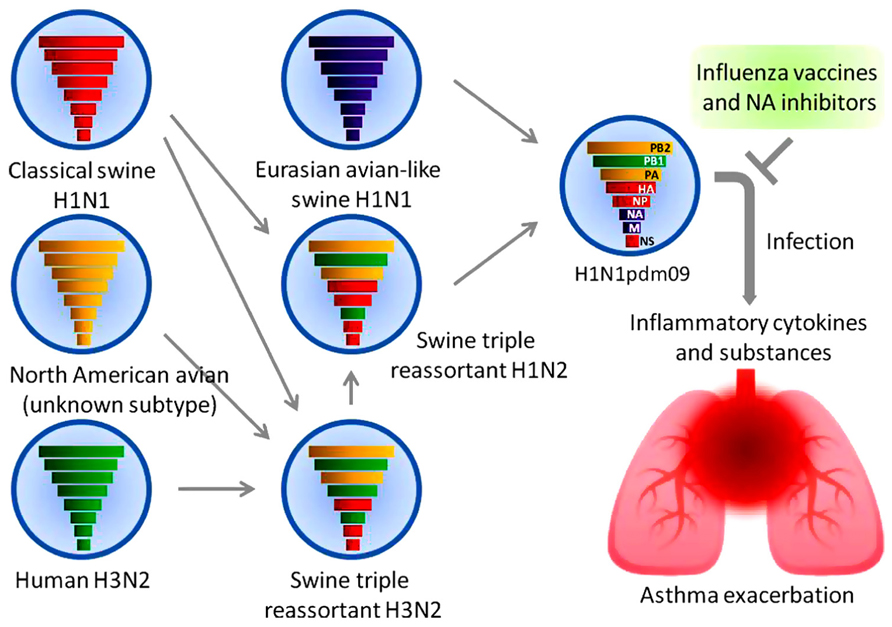
Importance of Influenza A (H1N1) Vaccination
Vaccination plays a crucial role in controlling the spread of the H1N1 virus. Immunization helps reduce the risk of severe illness, hospitalization, and mortality. It is especially vital for individuals in high-risk groups, including children, elderly individuals, pregnant women, and those with underlying medical conditions.
Types of Influenza A (H1N1) Vaccines
There are two primary types of H1N1 vaccines available:
1. Inactivated Influenza Vaccine (IIV)
- Administered via injection
- Contains a killed virus, ensuring no risk of infection
- Recommended for individuals aged six months and older
2. Live Attenuated Influenza Vaccine (LAIV)
- Administered as a nasal spray
- Contains a weakened virus strain
- Suitable for healthy individuals aged 2 to 49 years
Vaccine Effectiveness
The effectiveness of the H1N1 vaccine depends on multiple factors, such as age, health status, and virus strain. Annual vaccination updates are essential since influenza viruses constantly mutate.
Factors Influencing Vaccine Efficacy
- Immune Response: Younger individuals typically develop stronger immunity than older adults.
- Strain Match: Vaccines targeting circulating strains show higher effectiveness.
- Timing of Vaccination: Administering vaccines before peak flu season maximizes protection.
Recommended Vaccination Schedule
- Annual vaccination is advised, ideally before the onset of the flu season.
- Children under nine years receiving the vaccine for the first time may require two doses.
- Pregnant individuals can receive the vaccine at any stage of pregnancy to protect both the mother and the unborn child.
Side Effects and Safety
The influenza A (H1N1) vaccine is generally safe, but mild side effects may occur:
Common Side Effects
- Soreness, redness, or swelling at the injection site
- Mild fever or fatigue
- Headache and muscle aches
Rare Side Effects
- Severe allergic reactions (extremely uncommon)
- Guillain-Barré Syndrome (GBS), a rare neurological disorder, may develop in isolated cases
Who Should Not Receive the H1N1 Vaccine?
While most individuals benefit from vaccination, certain groups should consult a healthcare provider before immunization:
- Individuals with severe egg allergies
- Those with a history of severe allergic reactions to flu vaccines
- People diagnosed with Guillain-Barré Syndrome following previous vaccinations
Herd Immunity and Public Health Impact
Widespread vaccination helps achieve herd immunity, reducing the virus’s transmission across communities. Protecting vulnerable groups through herd immunity is essential in preventing outbreaks.
Storage and Handling of Vaccines
To maintain vaccine potency:
- Store vaccines between 2°C and 8°C
- Avoid freezing, as it compromises vaccine integrity
- Follow recommended handling protocols for transportation and administration
Vaccination against influenza A (H1N1) is a vital preventive measure to safeguard individuals and communities. Annual immunization is strongly recommended to ensure continued protection against evolving viral strains. Consult a healthcare provider to determine the most suitable vaccination option for you and your family.