Immunosuppression induction therapy is a critical component of lung transplantation, designed to prevent acute rejection and improve graft survival. This guide explores the types, mechanisms, and clinical considerations for effective immunosuppressive strategies in lung transplant recipients.
Importance of Immunosuppression in Lung Transplantation
Lung transplantation faces higher rejection rates compared to other solid organ transplants due to the lung’s extensive exposure to environmental factors. Effective immunosuppression induction therapy reduces the risk of acute and chronic rejection, ultimately enhancing patient outcomes.
Key Immunosuppressive Agents in Induction Therapy
Induction therapy typically involves a combination of powerful immunosuppressants to achieve optimal graft tolerance. Commonly used agents include:
1. Monoclonal and Polyclonal Antibodies
- Basiliximab: An IL-2 receptor antagonist that reduces T-cell activation with minimal nephrotoxicity.
- Alemtuzumab: Targets CD52 antigens on lymphocytes, providing potent and prolonged immunosuppression.
- Anti-Thymocyte Globulin (ATG): Derived from rabbit or horse serum, ATG depletes T-cells efficiently, lowering rejection risks.
2. Calcineurin Inhibitors (CNIs)
- Tacrolimus: A widely used CNI that inhibits T-cell activation, essential in early post-transplant care.
- Cyclosporine: An alternative to Tacrolimus, with a comparable efficacy profile.
3. Corticosteroids
- Methylprednisolone: Commonly administered intraoperatively to mitigate cytokine release and minimize inflammation.
Mechanism of Immunosuppression Induction Therapy
The goal of induction therapy is to suppress T-cell proliferation, block cytokine release, and reduce antibody production. The following diagram outlines the immunosuppression mechanism:
Protocols for Immunosuppression Induction Therapy
Protocols vary depending on patient risk factors, infection history, and underlying health conditions. Typical strategies include:
- High-risk patients: ATG combined with corticosteroids for aggressive T-cell depletion.
- Standard-risk patients: Basiliximab or Alemtuzumab with lower steroid doses to minimize side effects.
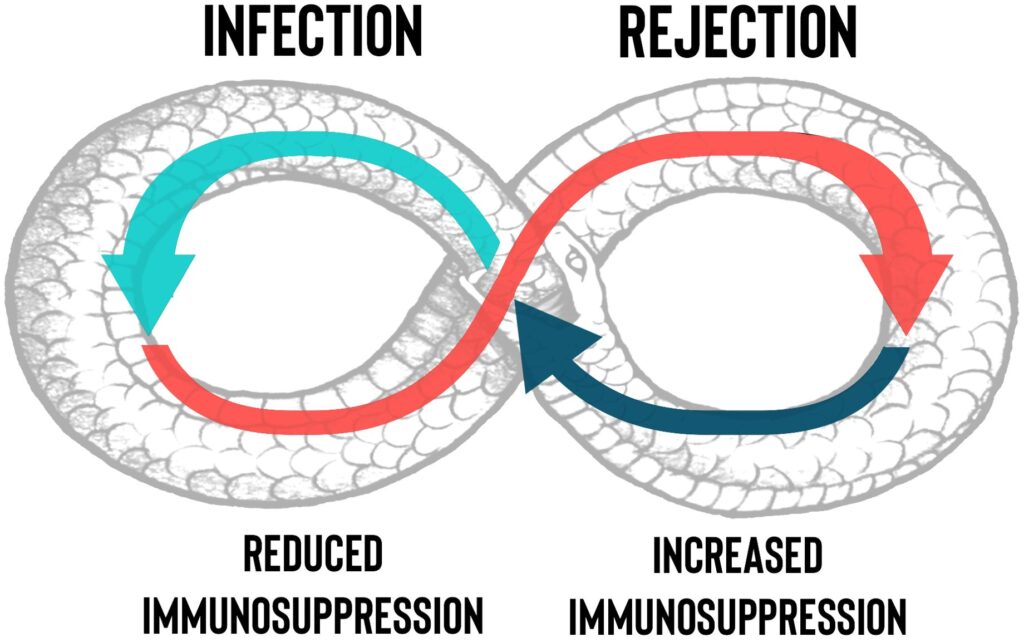
Monitoring and Managing Side Effects
Close monitoring is crucial to balance immune suppression and infection risks. Key considerations include:
- Infection control: Prophylactic antibiotics and antifungals reduce opportunistic infections.
- Renal function monitoring: CNIs are nephrotoxic, requiring routine kidney function assessment.
- Malignancy surveillance: Long-term immunosuppression may elevate cancer risks, necessitating regular screenings.
Long-Term Immunosuppression Maintenance
Following induction, patients transition to maintenance immunosuppression protocols involving CNIs, mycophenolate mofetil, and low-dose corticosteroids to sustain immune control.
Effective immunosuppression induction therapy is vital for lung transplantation success. By combining targeted agents and personalized protocols, clinicians can reduce rejection risks, improve survival rates, and enhance patient outcomes.
FAQs
Q1: What is the best induction therapy for high-risk lung transplant patients?
ATG combined with corticosteroids is the preferred choice for high-risk individuals due to its potent T-cell depletion effects.
Q2: How long should induction therapy last after lung transplantation?
Induction therapy typically lasts for the first few weeks to months post-transplant, followed by long-term maintenance therapy.
Q3: Are there risks of over-suppressing the immune system?
Yes, excessive immunosuppression can increase infection risks and malignancies, requiring careful dosing and monitoring.
Q4: Which immunosuppressive drug has the lowest risk of nephrotoxicity?
Basiliximab is known for its minimal nephrotoxic effects compared to CNIs.
Q5: How is immunosuppressive therapy adjusted over time?
Therapy is typically tapered down gradually, based on patient stability, infection control, and overall health.