High-grade gliomas (HGGs) are aggressive brain tumors characterized by rapid progression and resistance to conventional therapies. Among them, a subset harbors the BRAF V600E mutation, a genetic alteration with significant therapeutic implications. Understanding the molecular basis, diagnostic approaches, and treatment options for high-grade glioma with BRAF V600E mutation is crucial for improving patient outcomes.
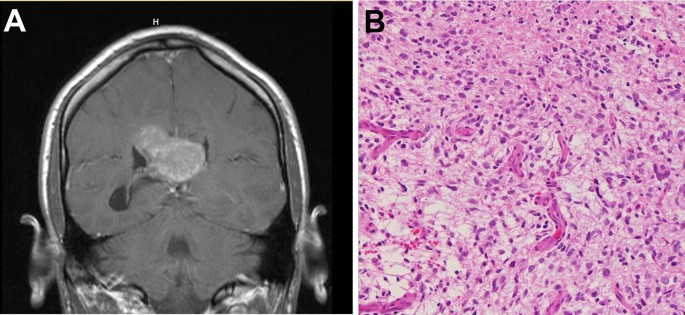
Pathogenesis and Molecular Characteristics
What is the BRAF V600E Mutation?
The BRAF V600E mutation results from a valine-to-glutamic acid substitution at codon 600 of the BRAF gene, leading to constitutive activation of the MAPK/ERK signaling pathway. This drives uncontrolled cell proliferation and survival, contributing to glioma pathogenesis.
Incidence in High-Grade Gliomas
The BRAF V600E mutation is found in approximately:
- 50-60% of pleomorphic xanthoastrocytomas (PXAs)
- 20-25% of anaplastic gangliogliomas
- 5-10% of glioblastomas (GBMs), particularly in pediatric and young adult cases
Role in Tumor Progression
HGGs with BRAF V600E mutations often exhibit:
- Increased tumor cell proliferation
- Enhanced angiogenesis
- Resistance to standard chemotherapy and radiation
Diagnosis and Molecular Testing
Imaging Features
MRI is the gold standard for diagnosing high-grade gliomas. Common imaging characteristics include:
- Irregular enhancement on contrast-enhanced MRI
- Necrotic and hemorrhagic components in advanced cases
- Infiltrative growth patterns
Histopathological and Molecular Analysis
Definitive diagnosis requires a biopsy with molecular profiling, including:
- Immunohistochemistry (IHC): Anti-BRAF V600E antibody staining
- Polymerase Chain Reaction (PCR): Detecting the specific BRAF V600E mutation
- Next-Generation Sequencing (NGS): Comprehensive genetic analysis to identify co-occurring mutations (e.g., IDH1/IDH2, TERT promoter mutations)
Treatment Strategies
Standard Treatment Approaches
- Surgical Resection:
- Maximal safe resection remains the primary treatment to reduce tumor burden.
- Extent of resection (EOR) significantly influences prognosis.
- Radiation Therapy:
- Fractionated external beam radiotherapy (EBRT) is standard.
- Stereotactic radiosurgery (SRS) may be considered for recurrent cases.
- Chemotherapy:
- Temozolomide (TMZ) is the first-line chemotherapy.
- Bevacizumab (anti-VEGF therapy) is used for recurrent cases.
Targeted Therapy for BRAF V600E-Mutant Gliomas
BRAF inhibitors and MEK inhibitors have shown promising results in treating gliomas with BRAF V600E mutations:
- Dabrafenib (BRAF inhibitor) + Trametinib (MEK inhibitor): Improved progression-free survival (PFS) in pediatric and adult patients.
- Vemurafenib: Used in cases resistant to conventional therapy.
Immunotherapy and Novel Approaches
- Checkpoint inhibitors (e.g., Pembrolizumab, Nivolumab): Under investigation in clinical trials.
- CAR-T Cell Therapy: A potential future treatment for gliomas.
Prognosis and Survival Outcomes
The prognosis for high-grade gliomas with BRAF V600E mutation varies:
- Pediatric and young adult patients tend to have better survival outcomes.
- BRAF inhibitors significantly improve progression-free survival (PFS) and overall survival (OS).
- Glioblastomas with BRAF V600E mutations remain challenging to treat, requiring multimodal therapy.
Key Prognostic Factors
- Extent of surgical resection
- Response to BRAF/MEK inhibitors
- MGMT promoter methylation status
Future Directions and Ongoing Research
Research continues to explore novel strategies:
- Combination therapies: BRAF inhibitors with immunotherapy.
- Personalized medicine: Precision oncology approaches.
- Gene editing: CRISPR-based strategies to target glioma cells.
High-grade gliomas with BRAF V600E mutation represent a distinct molecular subgroup with targeted treatment potential. Advances in genomic profiling and targeted therapy have significantly improved management strategies, offering better survival outcomes, especially in younger patients. Future research aims to optimize combination therapies and precision medicine approaches to further enhance patient prognosis.