Doxorubicin, a widely used anthracycline chemotherapeutic agent, is highly effective against various cancers but carries a significant risk of cardiotoxicity. This toxicity can lead to doxorubicin-induced cardiomyopathy (DIC), a potentially irreversible condition characterized by progressive heart failure. The cardiotoxic effects of doxorubicin are dose-dependent and can manifest acutely or years after treatment. Understanding the underlying mechanisms, early diagnostic markers, and optimal management strategies is crucial for improving patient outcomes.
Mechanisms of Doxorubicin-Induced Cardiotoxicity
Doxorubicin-induced cardiotoxicity is a complex process involving multiple interrelated mechanisms:
Oxidative Stress and Reactive Oxygen Species (ROS)
Doxorubicin undergoes redox cycling in cardiomyocytes, generating excessive ROS. The high metabolic demand of cardiac tissue makes it particularly susceptible to oxidative damage, leading to lipid peroxidation, mitochondrial dysfunction, and apoptosis. Unlike other tissues, cardiomyocytes have limited regenerative capacity, making ROS-mediated damage a significant contributor to DIC.
Topoisomerase IIβ (Top2β) Interaction
Doxorubicin binds to Top2β, an enzyme crucial for DNA repair and maintenance in cardiomyocytes. This interaction leads to DNA double-strand breaks, activation of stress pathways, and subsequent cardiomyocyte apoptosis. Top2β inhibition has been implicated in long-term cardiac dysfunction and fibrosis.
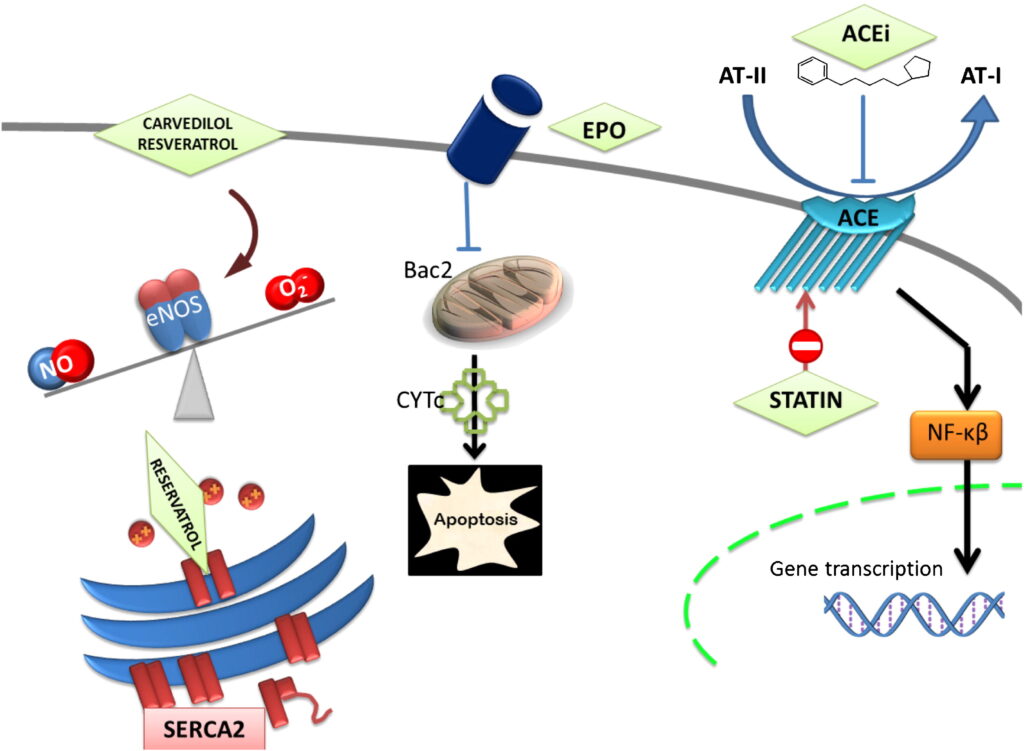
Mitochondrial Dysfunction
Doxorubicin impairs mitochondrial biogenesis and function, disrupting ATP production. Dysfunctional mitochondria further exacerbate ROS production, initiating a vicious cycle of oxidative stress, metabolic impairment, and cardiomyocyte apoptosis.
Calcium Dysregulation
Doxorubicin disrupts calcium homeostasis by affecting sarcoplasmic reticulum calcium handling. This leads to abnormal calcium cycling, impaired contractility, and increased susceptibility to arrhythmias.
Inflammation and Fibrosis
Inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukins, are upregulated in response to doxorubicin-induced damage. Persistent inflammation contributes to myocardial fibrosis, leading to ventricular stiffness and reduced contractile function.
mermaidCopyEditgraph LR
A[Doxorubicin] --> B[Oxidative Stress]
A --> C[Top2β Inhibition]
A --> D[Mitochondrial Dysfunction]
A --> E[Calcium Dysregulation]
B --> F[Cardiomyocyte Apoptosis]
C --> F
D --> F
E --> G[Arrhythmias]
F --> H[Heart Failure]
G --> H
Clinical Presentation and Diagnosis
Acute vs. Chronic Cardiotoxicity
- Acute cardiotoxicity: Occurs within hours to weeks of administration, presenting as transient arrhythmias, myocarditis, or pericarditis.
- Chronic cardiotoxicity: Develops months to years after treatment, progressing to dilated cardiomyopathy and congestive heart failure. The risk is significantly elevated at cumulative doses exceeding 400 mg/m².
Diagnostic Approaches
Echocardiography
- Left Ventricular Ejection Fraction (LVEF): A decline in LVEF is a key indicator of doxorubicin-induced cardiomyopathy.
- Global Longitudinal Strain (GLS): More sensitive than LVEF in detecting early myocardial dysfunction.
Cardiac Biomarkers
- Troponin I and Troponin T: Elevated levels correlate with myocardial injury and predict long-term cardiotoxicity.
- B-type Natriuretic Peptide (BNP) and NT-proBNP: Indicators of myocardial stress and heart failure development.
Advanced Imaging
- Cardiac Magnetic Resonance Imaging (CMR): Provides detailed myocardial characterization, detecting early fibrosis and inflammation.
- Radionuclide Angiography: Assesses ventricular function with high sensitivity.
Management Strategies
Preventive Approaches
Dose Limitation and Scheduling
Cumulative doxorubicin doses should be minimized while maintaining therapeutic efficacy. Fractionated dosing and continuous infusion reduce peak plasma concentrations, potentially mitigating toxicity.
Dexrazoxane
This iron-chelating agent reduces oxidative stress and DNA damage by limiting free radical formation. It is FDA-approved for cardioprotection in high-risk patients receiving doxorubicin.
Liposomal Doxorubicin
Encapsulation of doxorubicin in liposomes alters drug pharmacokinetics, reducing myocardial exposure and lowering the risk of cardiotoxicity.
Therapeutic Interventions
Pharmacologic Management
- Angiotensin-Converting Enzyme (ACE) Inhibitors & Angiotensin Receptor Blockers (ARBs): Reduce afterload, attenuate myocardial remodeling, and improve survival.
- Beta-Blockers (Carvedilol, Nebivolol): Decrease oxidative stress, reduce heart rate, and improve left ventricular function.
- Mineralocorticoid Receptor Antagonists (Spironolactone, Eplerenone): Provide antifibrotic benefits in heart failure patients.
Non-Pharmacologic Approaches
- Lifestyle Modifications: Sodium restriction, regular physical activity, and weight management are essential for heart failure prevention.
- Mechanical Circulatory Support: In advanced cases, ventricular assist devices (VADs) or heart transplantation may be necessary.