Diffuse astrocytoma is a grade II glioma that arises from astrocytes, the star-shaped glial cells that support and protect neurons. Unlike well-circumscribed tumors, these neoplasms diffusely infiltrate brain tissue, making complete surgical removal challenging. While considered low-grade, they can progress to more aggressive gliomas, such as anaplastic astrocytoma (grade III) or glioblastoma (grade IV).
Epidemiology and Risk Factors
- Represents 2-5% of all primary brain tumors.
- Typically diagnosed in individuals aged 20-50 years.
- Slight male predominance.
- Genetic predisposition plays a role, with mutations in TP53, ATRX, and IDH1/IDH2 genes frequently implicated.
- Prior radiation exposure is a known risk factor.
Pathophysiology and Molecular Classification
Cellular Origin and Growth Pattern
Diffuse astrocytomas originate from astrocytes and exhibit an infiltrative growth pattern, lacking well-defined tumor margins. This characteristic makes them more resistant to surgical resection.
Molecular Subtypes
The 2021 WHO Classification of CNS Tumors emphasizes molecular characteristics over histology alone:
- IDH-Mutant Diffuse Astrocytoma
- Accounts for the majority of cases.
- Associated with better prognosis.
- Often linked to TP53 and ATRX mutations.
- IDH-Wildtype Diffuse Astrocytoma
- Behaves more aggressively and resembles glioblastoma in progression.
- Worse prognosis compared to IDH-mutant counterparts.
Clinical Presentation
Symptoms depend on tumor location and rate of growth. Common signs include:
- Seizures – Often the first symptom.
- Headaches – Due to increased intracranial pressure.
- Cognitive Decline – Memory loss, personality changes, or impaired concentration.
- Motor or Sensory Deficits – Weakness, numbness, or difficulty with coordination.
Diagnostic Evaluation
Imaging Studies
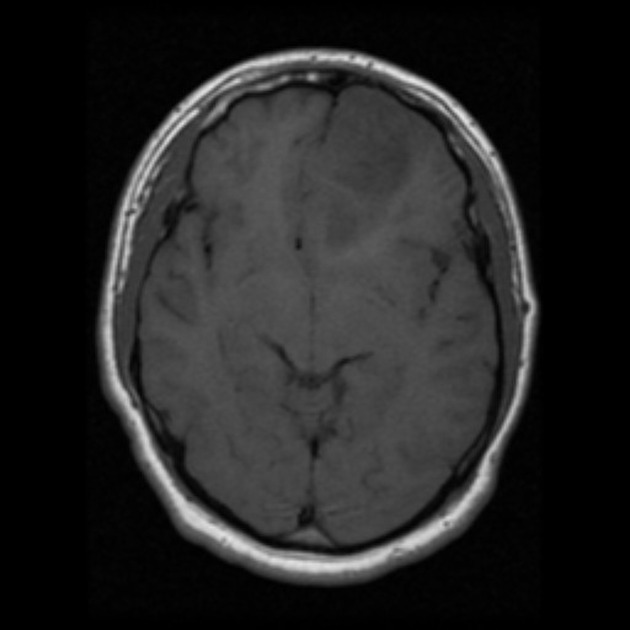
- Magnetic Resonance Imaging (MRI)
- Gold-standard for diagnosis.
- T2/FLAIR hyperintensity with no or minimal contrast enhancement.
- Magnetic Resonance Spectroscopy (MRS)
- Elevated choline and decreased N-acetylaspartate (NAA) suggest tumor presence.
Histopathology and Molecular Testing
- Biopsy or Resection Sample
- Low mitotic activity, no necrosis.
- Immunohistochemistry for IDH1 R132H mutation.
- Molecular Profiling
- IDH1/IDH2 mutation testing.
- ATRX loss correlates with astrocytic lineage.
Treatment Approaches
Surgical Resection
- Goal: Maximal safe tumor removal.
- Extent of Resection Matters:
- Gross total resection (GTR) improves progression-free survival.
- Subtotal resection (STR) requires adjunctive therapy.
Radiation Therapy
- Typically recommended after incomplete resection or tumor progression.
- Standard fractionation: 50-54 Gy over several weeks.
Chemotherapy
- Temozolomide (TMZ) – Frequently used in IDH-mutant astrocytomas.
- PCV Regimen (Procarbazine, Lomustine, Vincristine) – Alternative for selected cases.
Follow-Up and Surveillance
- MRI every 3-6 months to monitor recurrence.
- Long-term observation due to risk of progression to higher-grade gliomas.
Prognosis and Survival Rates
Factors affecting prognosis:
✅ IDH mutation status – IDH-mutant tumors have better survival.
✅ Extent of surgical resection – More extensive resection improves outcomes.
✅ Patient age and performance status – Younger patients fare better.
| Factor | Prognostic Impact |
|---|---|
| IDH-Mutant Status | Better prognosis |
| Complete Resection | Increased survival |
| Age <40 Years | Longer survival time |
| IDH-Wildtype Status | Poor prognosis |
Median survival:
- IDH-mutant astrocytoma: 8-12 years
- IDH-wildtype astrocytoma: <5 years
Progression to Higher-Grade Gliomas
Diffuse astrocytomas often progress to anaplastic astrocytoma (grade III) or glioblastoma (grade IV). Surveillance with periodic MRI scans is critical to detect early transformation.
Future Directions and Research
- Targeted Therapies – Ongoing trials for IDH inhibitors.
- Immunotherapy Approaches – Exploring checkpoint inhibitors for gliomas.
- Advanced Imaging Techniques – AI-driven MRI analysis for early detection.