Diamond-Blackfan Anemia (DBA) is a rare congenital bone marrow disorder characterized by insufficient production of red blood cells (RBCs). It is a type of inherited bone marrow failure syndrome (IBMFS), primarily affecting infants and young children. DBA occurs due to genetic mutations affecting ribosomal protein genes, leading to defective erythropoiesis.
Causes and Genetic Basis of DBA
DBA is predominantly caused by mutations in genes encoding ribosomal proteins. These mutations disrupt ribosome function, impairing the production of RBC precursors. Approximately 50-70% of cases have identifiable genetic mutations, with RPS19 being the most commonly implicated gene.
Key Genetic Factors:
- RPS19 (~25% of cases)
- RPL5, RPL11, RPL35A (less common)
- De novo mutations (spontaneous genetic changes in non-inherited cases)
DBA follows an autosomal dominant inheritance pattern, meaning a single mutated gene from one parent can cause the condition, although some cases arise sporadically.
Clinical Symptoms of Diamond-Blackfan Anemia
Patients with DBA typically exhibit symptoms within the first year of life. The severity varies, but common clinical signs include:
Hematological Symptoms:
- Severe macrocytic anemia (low RBC count with abnormally large RBCs)
- Reticulocytopenia (low reticulocyte count, indicating reduced RBC production)
- Normal or slightly low white blood cell and platelet counts
Non-Hematological Symptoms:
- Congenital anomalies (present in ~50% of DBA patients)
- Craniofacial abnormalities (cleft palate, microcephaly)
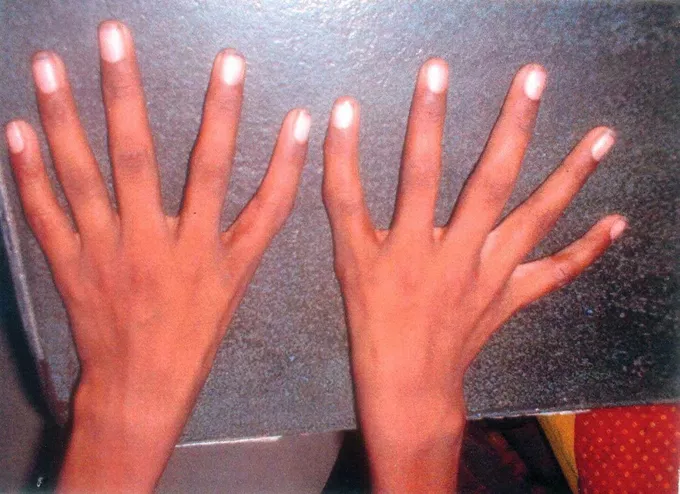
- Thumb malformations (triphalangeal thumbs, absent thumbs)
- Urogenital and cardiac defects
- Short stature and delayed growth
- Increased risk of cancer, including leukemia and osteosarcoma
Diagnosis of Diamond-Blackfan Anemia
DBA is diagnosed based on a combination of clinical presentation, laboratory findings, and genetic testing.
Diagnostic Criteria:
- Severe anemia within the first year of life
- Elevated erythrocyte adenosine deaminase (eADA) activity
- Bone marrow biopsy showing reduced erythroid precursors
- Genetic testing to identify ribosomal protein gene mutations
DBA must be differentiated from other bone marrow failure syndromes, such as Fanconi anemia and congenital dyserythropoietic anemia.
flowchart TD
A[Patient with suspected anemia] -->|Complete Blood Count (CBC)| B[Macrocytic anemia, low reticulocytes]
B -->|Bone Marrow Biopsy| C[Reduced erythroid precursors]
C -->|Genetic Testing| D[Mutation in ribosomal protein genes]
D -->|Diagnosis Confirmed| E[Diamond-Blackfan Anemia]Treatment and Management of DBA
While there is no universal cure for DBA, various treatments help manage the condition and improve quality of life.
1. Corticosteroid Therapy
- First-line treatment for most patients
- Prednisone or prednisolone helps stimulate RBC production
- Effective in ~80% of cases, but long-term use poses risks (osteoporosis, growth retardation, diabetes)
2. Chronic Blood Transfusions
- Used when steroids are ineffective or cause severe side effects
- Regular transfusions maintain hemoglobin levels but require iron chelation therapy to prevent iron overload
3. Hematopoietic Stem Cell Transplantation (HSCT)
- The only curative treatment for DBA
- Best results occur with a matched sibling donor
- Associated with risks, including graft-versus-host disease (GvHD)
4. Emerging Therapies and Research
- Gene therapy and targeted molecular treatments are under investigation
- L-leucine supplementation may stimulate erythropoiesis in some patients
Prognosis and Long-Term Outlook
With appropriate treatment, many DBA patients live into adulthood. However, they require lifelong monitoring due to risks of:
- Iron overload from transfusions
- Steroid-related complications
- Increased cancer risk
- Growth and developmental delays
Regular follow-ups with hematologists, endocrinologists, and genetic counselors are crucial for optimal care.