COVID-19, a global pandemic that has affected millions, continues to present challenges in terms of prevention and treatment. With new variants emerging and evolving strategies for managing exposure, post-exposure prophylaxis (PEP) has become a critical part of the public health response. This article explores the various facets of COVID-19 post-exposure prophylaxis (EUA), including emergency use authorizations (EUAs), treatments, and the protocols involved in minimizing the risk of severe outcomes following exposure to the virus.
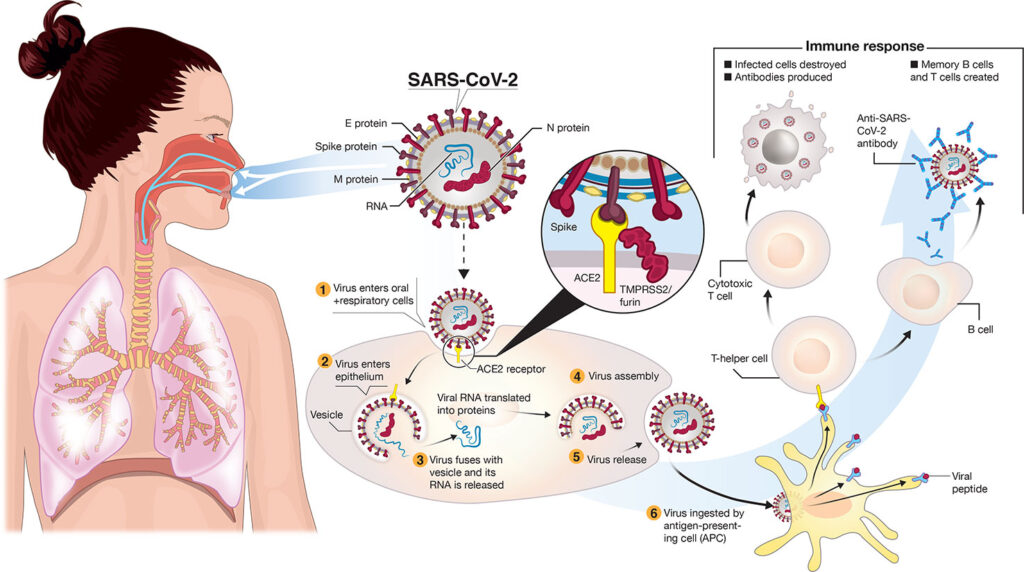
What is COVID-19 Post-Exposure Prophylaxis (PEP)?
COVID-19 post-exposure prophylaxis refers to preventive measures taken after a person has been exposed to the virus. The primary goal is to reduce the likelihood of infection or severe disease, particularly for individuals at high risk. These interventions are especially crucial for those who have been exposed to confirmed or suspected COVID-19 cases, where the virus may take time to incubate.
Post-exposure prophylaxis can include antiviral medications, monoclonal antibodies, and other treatments authorized under emergency use provisions, especially in individuals who are at increased risk due to underlying health conditions or their immunocompromised status.
Key Strategies in Post-Exposure Prophylaxis
- Antiviral Medications
Several antiviral treatments have been authorized for use in post-exposure settings. These treatments work by inhibiting the replication of the virus within the body, preventing the infection from spreading.
The most commonly used antiviral drugs include:- Paxlovid: A combination of nirmatrelvir and ritonavir, this medication is authorized for use in non-hospitalized patients with mild to moderate COVID-19 at high risk of progression to severe disease.
- Molnupiravir: Another oral antiviral authorized under EUA for COVID-19 treatment, molnupiravir is used for those at risk of severe disease but is typically less favored than Paxlovid due to its broader side-effect profile.
- Monoclonal Antibodies
Monoclonal antibody therapies are laboratory-made proteins that mimic the immune system’s ability to fight off harmful pathogens like COVID-19. These antibodies target the spike protein of the SARS-CoV-2 virus, effectively neutralizing it and preventing it from entering human cells.
Examples of authorized monoclonal antibody treatments include:- Bamlanivimab and Etesevimab: Used for post-exposure prevention and for early treatment in high-risk individuals.
- Casirivimab and Imdevimab: Also authorized for individuals exposed to COVID-19, especially in cases where vaccination alone may not provide sufficient protection.
- Pre-Exposure and Post-Exposure Vaccines
While vaccines are primarily a tool for preventing infection rather than post-exposure intervention, booster shots and vaccination regimens can serve as a form of prophylaxis in the event of exposure, especially when immunity from prior vaccination wanes.
Emergency Use Authorization (EUA) for COVID-19 Post-Exposure Prophylaxis
The U.S. Food and Drug Administration (FDA) has issued Emergency Use Authorizations (EUAs) for specific treatments in response to the COVID-19 pandemic. EUAs enable the distribution and administration of medical treatments before they receive full FDA approval, provided there is sufficient evidence suggesting their efficacy in preventing or treating COVID-19.
EUA for Antiviral Medications and Monoclonal Antibodies
Many of the antiviral and monoclonal antibody therapies used in COVID-19 post-exposure prophylaxis have been granted EUA, allowing for rapid deployment of these treatments during the pandemic. These EUAs are based on clinical data demonstrating that the benefits of the drugs outweigh the potential risks, especially when used in individuals at high risk for severe disease.
- Paxlovid and Molnupiravir received EUA for the treatment of COVID-19 in non-hospitalized patients who are at risk of severe outcomes.
- Monoclonal antibody treatments, like Bamlanivimab, Casirivimab, and Imdevimab, have also received EUAs for use in preventing infection post-exposure, especially in high-risk individuals such as those with compromised immune systems.
The EUA process helps accelerate the availability of these treatments, providing healthcare providers with critical tools for managing COVID-19 exposures.
Who Should Receive Post-Exposure Prophylaxis?
Post-exposure prophylaxis is particularly recommended for individuals who have had close contact with a confirmed COVID-19 case and are at increased risk of severe disease. High-risk populations include:
- Older adults: Those aged 65 years or older are at a significantly higher risk of developing severe COVID-19 symptoms.
- Immunocompromised individuals: People with weakened immune systems, such as those undergoing chemotherapy or organ transplant recipients.
- Individuals with chronic health conditions: People with heart disease, diabetes, or respiratory conditions may also be more vulnerable to COVID-19’s severe effects.
Post-exposure prophylaxis should be initiated as soon as possible after exposure to maximize its effectiveness. Timeliness is key in preventing the onset of symptoms and reducing the viral load.
Protocols for Administering Post-Exposure Prophylaxis
The administration of post-exposure prophylaxis should be carefully tailored to the individual, considering factors such as the severity of exposure, the individual’s health status, and the available treatment options. Healthcare providers follow guidelines set forth by the FDA, CDC, and other health organizations to ensure proper use.
Step-by-Step Guide:
- Assess Exposure Risk: A healthcare provider will assess the risk of exposure, considering the level of contact with an infected person and the individual’s health history.
- Determine Eligibility for PEP: Based on risk factors, eligibility for antiviral treatments or monoclonal antibodies will be determined.
- Initiate Treatment: If eligible, the healthcare provider will begin the appropriate course of treatment, whether oral antivirals or monoclonal antibodies.
- Monitor for Side Effects: Close monitoring for adverse reactions, especially in high-risk individuals, is essential.
- Follow-Up Care: Ongoing monitoring and follow-up appointments are necessary to ensure that the individual remains free from symptomatic COVID-19 and that there are no long-term complications.
The Role of COVID-19 Testing in Post-Exposure Prophylaxis
Testing plays a crucial role in identifying those who are infected after exposure, allowing healthcare providers to assess the need for post-exposure prophylaxis. Rapid antigen tests or PCR tests may be recommended for individuals who have been exposed to a confirmed case of COVID-19. Depending on the results, a healthcare provider can determine the appropriate treatment course and whether post-exposure prophylaxis is necessary.