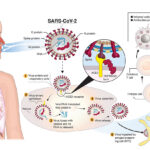
The COVID-19 pandemic has had an unparalleled impact on global health, leading to an urgent need for rapid solutions to combat the virus. One of the most significant measures in this effort has been the Emergency Use Authorization (EUA) process. This article delves into the nuances of EUA for COVID-19 treatments and vaccines, outlining its purpose, implementation, and effects on the pandemic response.
What is Emergency Use Authorization (EUA)?
Emergency Use Authorization (EUA) is a mechanism used by the U.S. Food and Drug Administration (FDA) that allows the use of medical products—such as vaccines, treatments, and diagnostic tests—that have not yet received full FDA approval, but are necessary in situations where there is a public health emergency. The EUA process was designed to facilitate faster access to critical medical products during urgent health crises like the COVID-19 pandemic.
For a product to receive an EUA, the FDA evaluates available scientific evidence to ensure that the product’s benefits outweigh any potential risks. While the process does not require the extensive trials and data collection required for full approval, the product must still demonstrate its safety and effectiveness to address the emergency at hand.
How Does EUA Apply to COVID-19 Vaccines?
The development of vaccines for COVID-19 was expedited using the EUA process to address the urgent need for widespread immunization. Several vaccine candidates, including those developed by Pfizer-BioNTech, Moderna, and Johnson & Johnson, received EUA after rigorous clinical trials showed that they were both safe and effective in preventing COVID-19 infection.
The EUA Vaccine Approval Process
The EUA approval for COVID-19 vaccines involved several stages:
- Preclinical Research: Scientists began by testing the vaccine candidates in laboratory settings and on animals to assess their safety and immune response.
- Clinical Trials: These vaccines went through three phases of human clinical trials, involving tens of thousands of volunteers. The trials were designed to assess the vaccines’ safety, efficacy, and potential side effects.
- FDA Evaluation: After completing the clinical trials, vaccine manufacturers submitted their findings to the FDA. The FDA reviewed the data to ensure the vaccines met the necessary safety and efficacy standards.
- EUA Issuance: Based on the evaluation, the FDA granted Emergency Use Authorization for the vaccines, allowing them to be distributed to the public during the ongoing pandemic.
It is important to note that while EUA allows vaccines to be distributed and used widely, it does not mean that these vaccines are permanently approved. Full approval requires additional studies and data collection beyond the scope of the emergency situation.
The Role of EUA in Expediting Public Health Measures
The EUA process played a crucial role in expediting the distribution of COVID-19 vaccines and treatments. Without this emergency mechanism, it would have taken significantly longer for effective vaccines and therapeutics to reach the public.
Faster Response to Emerging Health Threats
The EUA allowed health authorities to rapidly deploy vaccines to vulnerable populations, such as healthcare workers and elderly individuals, who were at higher risk of severe COVID-19 disease. By making these treatments available more quickly, the EUA helped reduce the strain on healthcare systems and minimized fatalities during the peak of the pandemic.
Supporting Global Immunization Efforts
In addition to the U.S., other countries also adopted similar mechanisms to approve COVID-19 vaccines and treatments, leading to a global push for vaccination. The widespread use of EUA-facilitated vaccines has contributed to the ongoing effort to mitigate the effects of COVID-19 worldwide.
Potential Risks and Challenges of EUA
While the EUA process is essential in responding to health emergencies, it does come with challenges and potential risks.
Limited Data on Long-Term Effects
Because the clinical trials for EUA-approved vaccines were expedited, long-term data on their safety and effectiveness may not be as extensive as that for fully approved vaccines. This limitation means that ongoing monitoring and surveillance are necessary to ensure the continued safety of the vaccines in the population.
Public Trust and Perception
The use of EUA can sometimes create public skepticism, especially when it comes to newly developed medical products. Some individuals may perceive the EUA process as rushed or insufficiently rigorous, which can hinder vaccination efforts. Public education and transparent communication are key to addressing these concerns and ensuring widespread adoption of vaccines.
EUA for COVID-19 Treatments and Diagnostic Tests
In addition to vaccines, the EUA process has been used to approve several COVID-19 treatments and diagnostic tests. These include antiviral medications like Remdesivir, monoclonal antibody treatments, and rapid COVID-19 tests. Each of these products underwent rigorous evaluation to ensure they were effective in managing the disease or providing timely diagnosis, even though they did not yet have full FDA approval.
COVID-19 Diagnostic Tests
With the rapid spread of COVID-19, the ability to diagnose infections quickly became essential. EUA allowed for the approval of diagnostic tests, including PCR and antigen tests, that were crucial for widespread testing efforts. These tests helped identify cases, reduce transmission, and guide quarantine measures.
Therapeutic Treatments
The EUA also facilitated the rapid deployment of treatments to manage severe COVID-19 cases. Drugs such as Remdesivir and monoclonal antibodies were authorized under the EUA process, providing healthcare providers with effective options to treat hospitalized patients and reduce the severity of the disease.
Monitoring and Safety Measures Post-EUA
After a product is authorized under the EUA, it is still subject to ongoing monitoring. This includes tracking any adverse events or side effects through systems like the Vaccine Adverse Event Reporting System (VAERS) and the FDA’s MedWatch. These surveillance mechanisms are crucial for ensuring that any unforeseen risks are promptly identified and addressed.
Continued Research and Data Collection
Even after a product is granted EUA, additional clinical trials and research are conducted to gather more information about its long-term effects and broader population safety. If any concerns arise, the FDA has the authority to revoke the EUA or impose additional restrictions on the product.
Future of Emergency Use Authorization
As the pandemic continues to evolve, the use of Emergency Use Authorization will likely remain a critical tool in addressing emerging health crises. However, as more data becomes available and as the COVID-19 situation stabilizes, many of the products authorized under EUA will transition to full FDA approval once the comprehensive data needed for such approval has been gathered.