Acute Myeloid Leukemia with Isocitrate Dehydrogenase-2 (IDH2) Mutation is a complex and aggressive hematologic malignancy characterized by the uncontrolled proliferation of abnormal myeloid cells. Among the numerous genetic mutations linked to AML, mutations in the isocitrate dehydrogenase 2 (IDH2) gene have gained prominence due to their significant role in leukemogenesis and their impact on prognosis and treatment response. This article explores the biological significance of IDH2 mutations in AML, their clinical implications, diagnostic methods, and current therapeutic strategies.
Understanding Acute Myeloid Leukemia with Isocitrate Dehydrogenase-2 (IDH2) Mutation
What is IDH2?
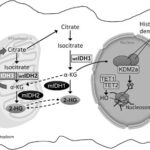
The IDH2 gene encodes an enzyme located in the mitochondria that plays a crucial role in the citric acid cycle by converting isocitrate into alpha-ketoglutarate (α-KG). This reaction is essential for cellular metabolism and epigenetic regulation. However, mutations in IDH2 lead to a neomorphic enzymatic activity, resulting in the abnormal production of D-2-hydroxyglutarate (2-HG), an oncometabolite that promotes leukemia development.
Prevalence of IDH2 Mutations in AML
- IDH2 mutations occur in 8%–12% of AML cases.
- More commonly observed in older adults.
- Often coexists with other genetic abnormalities such as NPM1 or FLT3 mutations, influencing treatment response.
Pathophysiology of IDH2 Mutations in AML
Mutant IDH2 converts α-KG into 2-HG, an aberrant metabolite that:
- Inhibits α-KG–dependent enzymes, disrupting normal cellular differentiation.
- Leads to epigenetic alterations, resulting in DNA hypermethylation.
- Blocks hematopoietic differentiation, trapping myeloid progenitors in an undifferentiated state, which contributes to AML development.
Clinical Significance and Prognosis
- IDH2 mutations influence the prognosis of AML, often leading to worse outcomes when combined with high-risk cytogenetics.
- Patients with isolated IDH2 mutations may exhibit a moderate response to standard chemotherapy.
- Minimal Residual Disease (MRD) monitoring is crucial for patients undergoing treatment.
Diagnosis of IDH2 Mutations
Accurate detection of IDH2 mutations is essential for prognosis and treatment selection. The following diagnostic methods are commonly used:
Molecular Testing Approaches
- Polymerase Chain Reaction (PCR): Rapidly identifies specific IDH2 mutations.
- Next-Generation Sequencing (NGS): Provides a comprehensive genetic profile of AML.
- Liquid Biopsy: Enables non-invasive detection of circulating tumor DNA.
- Immunohistochemistry (IHC): Detects mutant IDH2 protein expression in bone marrow samples.
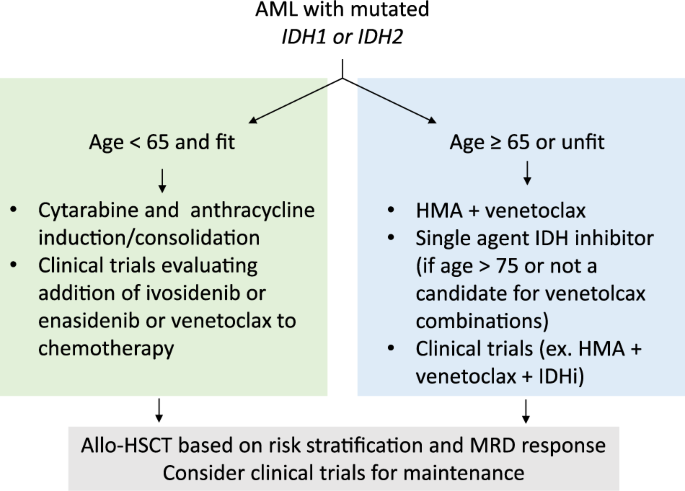
Treatment Strategies for IDH2-Mutated AML
1. Targeted Therapy with IDH2 Inhibitors
The development of IDH2 inhibitors has significantly improved treatment options for patients with AML harboring this mutation.
Enasidenib (IDHIFA®)
- Mechanism: Selectively inhibits mutant IDH2, reducing 2-HG levels and allowing differentiation of leukemic cells.
- Indication: Approved for relapsed or refractory AML with IDH2 mutations.
- Clinical Benefit: Improves overall survival and complete remission (CR) rates in selected patients.
2. Chemotherapy and Combination Therapies
- Standard induction chemotherapy (7+3 regimen) remains a primary treatment approach.
- Combining enasidenib with hypomethylating agents (HMAs) (e.g., azacitidine) has shown promising results.
3. Hematopoietic Stem Cell Transplantation (HSCT)
- Considered for high-risk patients who achieve remission with IDH2 inhibitors or chemotherapy.
- Improves long-term survival in eligible patients.
Future Directions in IDH2-Mutated AML Treatment
- Combination Therapies: Investigating the efficacy of IDH2 inhibitors with BCL-2 inhibitors (e.g., venetoclax).
- Next-Generation IDH2 Inhibitors: Developing more potent and selective drugs to overcome resistance.
- Biomarker Research: Identifying predictive biomarkers for therapy response and resistance mechanisms.
IDH2 mutations play a critical role in the pathogenesis of acute myeloid leukemia, influencing both prognosis and treatment responses. The introduction of IDH2 inhibitors such as enasidenib has transformed treatment paradigms, providing effective targeted therapy for relapsed or refractory AML. Continued research into combination therapies, biomarkers, and novel inhibitors holds promise for improving patient outcomes and long-term survival in AML with IDH2 mutations.