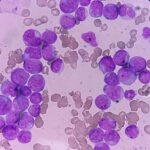
Acute Myeloid Leukemia with FLT3 Mutation is a hematologic malignancy characterized by the uncontrolled proliferation of myeloid precursor cells. Among its genetic mutations, the FMS-like tyrosine kinase 3 (FLT3) mutation is one of the most clinically significant, associated with a poor prognosis and high relapse rates. Understanding the molecular biology, diagnostic methods, and treatment strategies for FLT3-mutated AML is essential for improving patient outcomes.
Understanding FLT3 Mutations in AML
FLT3 mutations occur in approximately 30% of AML cases and primarily manifest in two forms:
- FLT3-ITD (Internal Tandem Duplication): Found in 25% of AML cases, it leads to constitutive activation of FLT3, promoting uncontrolled cell growth and survival.
- FLT3-TKD (Tyrosine Kinase Domain Mutation): Less common (5–10% of cases), it enhances signaling activity but with less aggressive clinical implications.
The FLT3-ITD mutation is particularly concerning due to its strong association with higher relapse rates and reduced overall survival.
Symptoms of FLT3-Mutated AML
Patients with FLT3-mutated AML may present with symptoms that overlap with other subtypes of AML, including:
- Persistent fatigue and weakness
- Frequent infections due to neutropenia
- Easy bruising and bleeding from thrombocytopenia
- Shortness of breath
- Bone pain
- Hepatosplenomegaly (enlarged liver and spleen)
Diagnosis and Molecular Testing
Standard Diagnostic Procedures
- Complete Blood Count (CBC) and Peripheral Blood Smear: Identifies blasts and cytopenias.
- Bone Marrow Biopsy: Confirms AML diagnosis and blast percentage.
- Flow Cytometry: Detects aberrant surface markers on AML cells.
Genetic and Molecular Testing
- Polymerase Chain Reaction (PCR) and Next-Generation Sequencing (NGS): Identifies FLT3-ITD and FLT3-TKD mutations.
- Cytogenetics and Fluorescence In Situ Hybridization (FISH): Detects co-occurring mutations that may impact treatment decisions.
- Minimal Residual Disease (MRD) Monitoring: Evaluates response to therapy and risk of relapse.
Treatment Strategies for FLT3-Mutated AML
1. Standard Induction Chemotherapy
- 7+3 Regimen: Combination of cytarabine (7 days) and daunorubicin or idarubicin (3 days).
- Intensive Chemotherapy remains the standard first-line treatment, though FLT3 inhibitors have significantly improved survival rates when added.
2. Targeted Therapy with FLT3 Inhibitors
FLT3 inhibitors have revolutionized the treatment landscape:
- Midostaurin: Approved for newly diagnosed patients in combination with chemotherapy.
- Gilteritinib: Used for relapsed/refractory FLT3-mutated AML.
- Quizartinib and Crenolanib: Under investigation for broader efficacy.
3. Hematopoietic Stem Cell Transplantation (HSCT)
- Recommended for high-risk patients, especially those with FLT3-ITD mutations.
- Allogeneic HSCT improves long-term survival in eligible patients.
4. Combination Therapies and Emerging Treatments
- BCL-2 inhibitors (e.g., Venetoclax) in combination with FLT3 inhibitors show promise.
- Immunotherapy and CAR-T cell approaches are under investigation.
Prognosis and Survival Rates
FLT3-mutated AML has historically been associated with poor outcomes:
- FLT3-ITD mutation: 5-year survival rate of ~20-30% without targeted therapy.
- FLT3 inhibitors + HSCT: Improved survival, especially in younger patients.
- Relapsed/Refractory AML: Lower survival, but novel therapies offer hope.
Future Directions and Ongoing Research
- Development of next-generation FLT3 inhibitors with fewer resistance mechanisms.
- Combination strategies targeting multiple pathways for better disease control.
- Personalized medicine approaches using genetic profiling for tailored treatments.
Acute myeloid leukemia with FLT3 mutation is an aggressive disease requiring rapid diagnosis and targeted therapy. Advances in FLT3 inhibitors, stem cell transplantation, and novel drug combinations have significantly improved survival outcomes. Ongoing research continues to refine treatment options, offering hope for better management and increased life expectancy for affected patients.