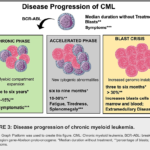
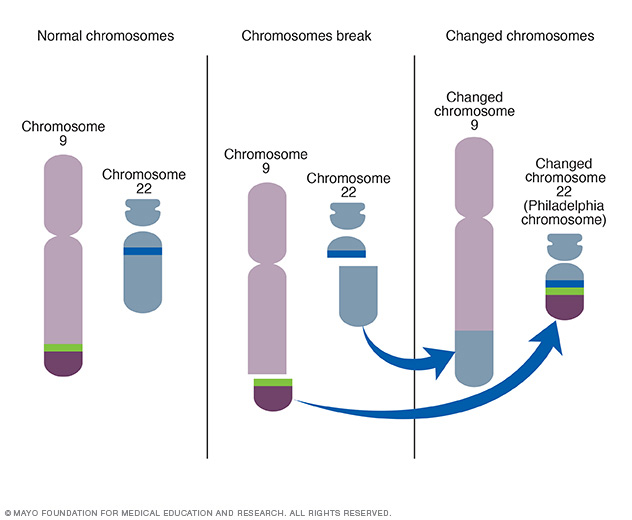
Accelerated phase philadelphia chromosome positive chronic myelocytic leukemia (CML) is a myeloproliferative disorder characterized by the presence of the BCR-ABL fusion gene, a result of a translocation between chromosomes 9 and 22. The disease progresses through three stages: chronic phase, accelerated phase, and blast crisis. This article focuses on the accelerated phase of Philadelphia chromosome-positive CML, exploring its diagnosis, pathophysiology, clinical features, and treatment strategies.
Understanding the Accelerated Phase of CML
The accelerated phase is an intermediate stage between the chronic phase and the advanced blast crisis. During this phase, the disease becomes more aggressive, with an increase in leukemic cell burden and resistance to treatment. Early identification and prompt intervention are critical to improving patient outcomes.
Criteria for Diagnosing the Accelerated Phase
The World Health Organization (WHO) and the European LeukemiaNet have established diagnostic criteria for the accelerated phase. Common features include:
- Blast Count: 10%–19% blasts in the peripheral blood or bone marrow.
- Basophils: Elevated basophils, typically exceeding 20% in the blood.
- Platelet Abnormalities: Persistent thrombocytopenia (<100,000/μL) or thrombocytosis (>1,000,000/μL) unresponsive to treatment.
- Cytogenetic Evolution: Additional chromosomal abnormalities beyond the Philadelphia chromosome.
- Worsening Symptoms: Progressive splenomegaly and increasing white blood cell counts, despite therapy.
Pathophysiology of the Accelerated Phase
The accelerated phase represents increased genomic instability in leukemic cells. The BCR-ABL fusion gene produces the BCR-ABL tyrosine kinase, driving uncontrolled proliferation of myeloid cells. During the accelerated phase, mutations in the ABL kinase domain and other genetic alterations accumulate, leading to reduced sensitivity to tyrosine kinase inhibitors (TKIs).
Key Genetic and Molecular Alterations
- ABL Kinase Domain Mutations: Point mutations, such as T315I, are associated with resistance to first- and second-generation TKIs.
- Clonal Evolution: Additional cytogenetic abnormalities, such as trisomy 8, isochromosome 17q, or loss of the Y chromosome, signify disease progression.
- Increased Blast Population: Enhanced self-renewal capabilities of leukemic progenitors contribute to a higher blast percentage.
Clinical Features of Accelerated Phase CML
Patients in the accelerated phase often experience a marked decline in clinical status compared to the chronic phase. Symptoms may include:
- Fatigue: Resulting from anemia and the increased metabolic demands of leukemic cells.
- Fever and Night Sweats: Indicative of systemic inflammation.
- Bone Pain: Due to leukemic infiltration of the bone marrow.
- Splenomegaly: Progressive enlargement of the spleen, causing abdominal discomfort.
- Uncontrolled Leukocytosis: Persistently high white blood cell counts that are refractory to standard treatment.
Diagnostic Workup
Accurate diagnosis of the accelerated phase requires a comprehensive evaluation of clinical, hematologic, and molecular parameters.
Laboratory and Imaging Studies
- Complete Blood Count (CBC): Elevated white blood cell count, increased blasts, and basophilia.
- Bone Marrow Biopsy: Hypercellular marrow with increased blasts and dysplasia.
- Cytogenetic Analysis: Identification of additional chromosomal abnormalities via karyotyping or fluorescence in situ hybridization (FISH).
- Molecular Testing: Detection of BCR-ABL mutations using polymerase chain reaction (PCR) and sequencing.
Treatment Strategies for Accelerated Phase CML
The management of accelerated phase CML focuses on controlling disease progression, reducing the leukemic burden, and minimizing resistance. Therapy should be individualized based on patient characteristics, mutation status, and treatment response.
1. Tyrosine Kinase Inhibitors (TKIs)
TKIs are the cornerstone of treatment for Philadelphia chromosome-positive CML. Options for the accelerated phase include:
- Second-Generation TKIs: Nilotinib and dasatinib are preferred for their increased potency and ability to overcome resistance seen with imatinib.
- Third-Generation TKIs: Ponatinib is effective in patients with T315I mutations or resistance to other TKIs.
2. Combination Therapies
Combining TKIs with other therapeutic agents can improve outcomes. These include:
- Interferon-alpha: Enhances the immune response against leukemic cells.
- Cytotoxic Chemotherapy: Used to reduce the leukemic burden in cases of high blast counts.
3. Allogeneic Stem Cell Transplantation (SCT)
Allogeneic SCT remains the only curative option for CML. It is typically reserved for younger patients with a suitable donor or those who fail TKI therapy.
4. Clinical Trials
Participation in clinical trials exploring novel agents, such as ABL kinase inhibitors, BCL-2 inhibitors, or CAR-T cell therapy, may offer additional treatment avenues for refractory cases.
Prognosis and Monitoring
The prognosis for accelerated phase CML has improved significantly with the advent of TKIs. However, regular monitoring is essential to assess treatment response and detect disease progression.
Response Monitoring Guidelines
- Hematologic Response: Normalization of blood counts and resolution of splenomegaly.
- Cytogenetic Response: Reduction or elimination of Philadelphia chromosome-positive cells.
- Molecular Response: Decline in BCR-ABL transcript levels, measured by quantitative PCR.
The accelerated phase of Philadelphia chromosome-positive CML represents a critical juncture in disease progression. Prompt recognition and a tailored approach to treatment are essential for achieving optimal outcomes. Advances in TKIs and other therapies have transformed the prognosis for patients, offering hope for long-term disease control and improved quality of life. By understanding the complexities of the accelerated phase and implementing evidence-based interventions, healthcare professionals can provide the best possible care to patients facing this challenging stage of CML.