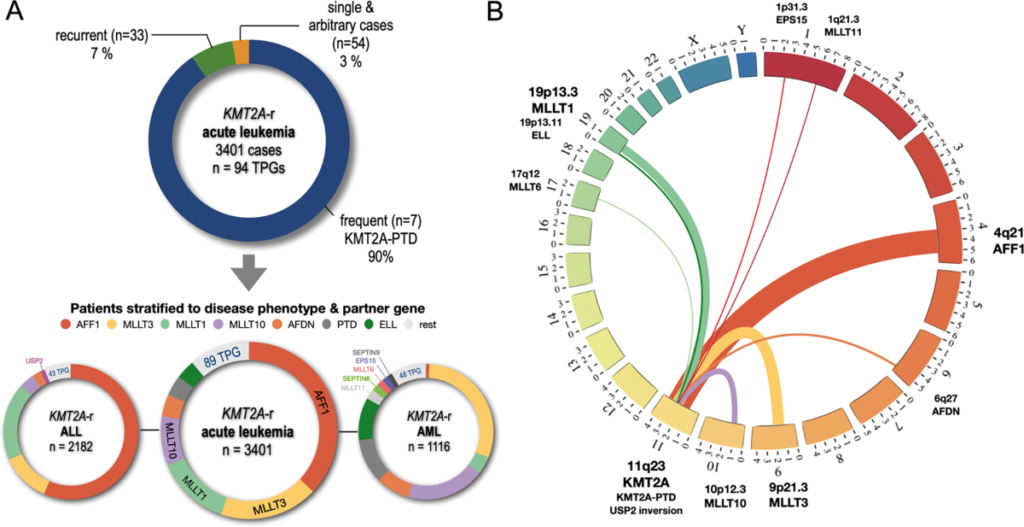
The lysine methyltransferase 2A gene (KMT2A), formerly known as MLL, plays a critical role in regulating histone H3K4 methylation, a process essential for transcriptional activation. Translocations involving KMT2A are recurrent genetic abnormalities observed in 5–10% of acute leukemias, including acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). Over 100 fusion partners have been identified, with common partners including AF4 (AFF1), AF9 (MLLT3), and ENL (MLLT1). These rearrangements disrupt epigenetic regulation, leading to aberrant expression of HOX genes and MEIS1, which drive leukemic transformation.
Clinical and Prognostic Implications of KMT2A-Rearranged Leukemia
KMT2A-rearranged leukemias are associated with aggressive disease phenotypes and poor outcomes. In infants, KMT2A-AF4 fusions account for 70% of ALL cases, with a 5-year survival rate below 50%. Adults with KMT2A-AML exhibit resistance to conventional chemotherapy, with median survival rarely exceeding 12 months. Key clinical features include hyperleukocytosis, central nervous system involvement, and rapid relapse post-remission.
Diagnostic Strategies for Detecting KMT2A Translocations
Accurate identification of KMT2A translocations requires multimodal approaches:
- Cytogenetic Analysis: Karyotyping detects large structural abnormalities but may miss cryptic rearrangements.
- Fluorescence In Situ Hybridization (FISH): A gold standard for diagnosing KMT2A breaks using dual-color probes.
- Next-Generation Sequencing (NGS): Captures partner genes and coexisting mutations (e.g., FLT3, RAS).
- RT-PCR: Validates fusion transcripts in ambiguous cases.
Targeted Therapies for KMT2A-Rearranged Leukemia
Current treatment paradigms are evolving to address KMT2A vulnerabilities:
- Epigenetic Modulators: DOT1L inhibitors (e.g., pinometostat) disrupt KMT2A-AF9-mediated histone methylation.
- Menin-KMT2A Interaction Blockers: Revumenib (SNDX-5613) shows promise in phase I/II trials, achieving complete remission in 30% of relapsed/refractory cases.
- Immunotherapy: CD19-directed CAR-T cells and bispecific antibodies (blinatumomab) are under investigation for KMT2A-ALL.
Future Directions in Research and Clinical Trials
Emerging biomarkers, such as KMT2A minimal residual disease (MRD) monitoring via droplet digital PCR, may refine risk stratification. Collaborative consortia, including the Children’s Oncology Group (COG) and Beat AML, are evaluating combination therapies (e.g., venetoclax + hypomethylating agents) to overcome therapeutic resistance.
KMT2A-rearranged leukemias represent a high-risk subgroup demanding precision medicine approaches. Advances in epigenetic targeting and immunotherapy offer hope for improving survival outcomes, underscoring the need for early molecular diagnostics and enrollment in clinical trials.